Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells
- PMID: 9916058
- PMCID: PMC96354
- DOI: 10.1128/IAI.67.2.554-561.1999
Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells
Abstract
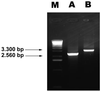
Most Klebsiella pneumoniae clinical isolates are fully encapsulated and adhere in vitro to intestinal cell lines with an aggregative pattern. In this study, the influence of the capsule on interactions with epithelial cells was investigated by creating an isogenic mutant defective in the synthesis of the capsule. Determination of the uronic acid content of bacterial extracts confirmed that the mutant did not produce capsular polysaccharides whereas, with the wild-type strain, the level of encapsulation was growth phase dependent and reached a maximum during the lag and early log phases. Assays performed with different epithelial cell lines, Int-407, A-549, and HEp-2, showed that the capsule-defective mutant demonstrated greater adhesion than did the wild-type strain and that the aggregative pattern was maintained, indicating that the capsule was not related to the adhesion phenotype. In contrast, when the mucus-producing HT-29-MTX cells were used, the encapsulated wild-type strain adhered more strongly than did the capsule-defective mutant. No invasion properties were observed with any of the capsular phenotypes or cell lines used. The K. pneumoniae adhesin CF29K was detected by Western blot analysis and enzyme-linked immunosorbent assay on the surface of transconjugants obtained after transfer of a conjugative plasmid harboring the CF29K-encoding genes into both the wild-type and the capsule-defective strains. The amounts of adhesin detected were greater in the capsule-defective background strain than in the wild-type encapsulated strain and were associated with an increase in the level of adhesion to Caco-2 cells. Moreover, RNA slot blot experiments showed that transcription of the adhesin-encoding gene was markedly increased in the capsule-defective mutant compared to the wild-type encapsulated background. These results suggest (i) that the capsule plays an active role during the initial steps of the pathogenesis by interacting with mucus-producing cells but is subsequently not required for the adhesin-related interaction with the epithelial cell surface and (ii) that the expression of the adhesin is modulated by the presence of a capsule at a transcriptional level.
Figures


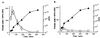

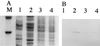
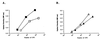

Similar articles
-
Influence of capsule and extended-spectrum beta-lactamases encoding plasmids upon Klebsiella pneumoniae adhesion.Res Microbiol. 2007 May;158(4):339-47. doi: 10.1016/j.resmic.2007.02.005. Epub 2007 Mar 3. Res Microbiol. 2007. PMID: 17446046
-
Molecular characterization and adhesive properties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections.Infect Immun. 1995 Nov;63(11):4336-44. doi: 10.1128/iai.63.11.4336-4344.1995. Infect Immun. 1995. PMID: 7591068 Free PMC article.
-
Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice.Infect Immun. 1999 Nov;67(11):6152-6. doi: 10.1128/IAI.67.11.6152-6156.1999. Infect Immun. 1999. PMID: 10531279 Free PMC article.
-
Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae.Infect Immun. 2000 Dec;68(12):6744-9. doi: 10.1128/IAI.68.12.6744-6749.2000. Infect Immun. 2000. PMID: 11083790 Free PMC article.
-
R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections.Infect Immun. 1992 Jan;60(1):44-55. doi: 10.1128/iai.60.1.44-55.1992. Infect Immun. 1992. PMID: 1345909 Free PMC article.
Cited by
-
Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process.Infect Immun. 2002 Jun;70(6):3180-6. doi: 10.1128/IAI.70.6.3180-3186.2002. Infect Immun. 2002. PMID: 12011013 Free PMC article.
-
Recognition of bacterial surface polysaccharides by lectins of the innate immune system and its contribution to defense against infection: the case of pulmonary pathogens.Infect Immun. 2008 Apr;76(4):1322-32. doi: 10.1128/IAI.00910-07. Epub 2007 Dec 17. Infect Immun. 2008. PMID: 18086817 Free PMC article. No abstract available.
-
Characterization of a DHA-1-producing Klebsiella pneumoniae strain involved in an outbreak and role of the AmpR regulator in virulence.Antimicrob Agents Chemother. 2012 Jan;56(1):288-94. doi: 10.1128/AAC.00164-11. Epub 2011 Oct 10. Antimicrob Agents Chemother. 2012. PMID: 21986829 Free PMC article.
-
Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells.Infect Immun. 2009 Feb;77(2):714-24. doi: 10.1128/IAI.00852-08. Epub 2008 Nov 17. Infect Immun. 2009. PMID: 19015258 Free PMC article.
-
Rhinoscleroma pathogenesis: The type K3 capsule of Klebsiella rhinoscleromatis is a virulence factor not involved in Mikulicz cells formation.PLoS Negl Trop Dis. 2018 Jan 30;12(1):e0006201. doi: 10.1371/journal.pntd.0006201. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29381692 Free PMC article.
References
-
- Allen P, Hart C, Saunders J. Isolation from Klebsiella and characterization of two rcs genes that activate colanic acid capsular biosynthesis in Escherichia coli. J Gen Microbiol. 1987;133:331–340. - PubMed
-
- Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey P S, Popoff M Y. The RcsB-RscC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. - PubMed
-
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. - PubMed
-
- Brosius J, Ullrich A, Raker M A, Gray A, Dull T J, Gutell R R, Noller H F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981;6:112–118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

