Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O
- PMID: 9916060
- PMCID: PMC96356
- DOI: 10.1128/IAI.67.2.568-575.1999
Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O
Abstract
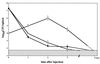
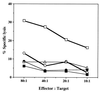
Only listeriolysin O (LLO)-producing strains of Listeria monocytogenes generate protective immunity in mice. Based on the findings that endogenous gamma interferon (IFN-gamma) production was induced only by such strains and that purified LLO could induce IFN-gamma from NK cells, we have postulated that LLO may play a pivotal role in the induction of Th1-type protective T cells, which are highly dependent on IFN-gamma. In this study, mice were immunized with L. monocytogenes ATCC 15313, an LLO-nonproducing avirulent strain, along with LLO encapsulated in liposome (LLO-liposome). LLO-liposome was highly potent in the induction of various cytokines, including IFN-gamma. Immunization of mice with either LLO-liposome or the viable strain ATCC 15313 alone did not induce protection against challenge infection. In contrast, the combination of LLO-nonproducing bacteria plus LLO-liposome induced a significant level of protective immunity mediated mainly by Th1-type cells capable of producing a large amount of IFN-gamma in an antigen-specific manner. The protection afforded by the combination was not dependent on LLO-specific cytotoxic T cells. These results support the idea that the inability of an LLO-nonproducing avirulent strain or killed bacteria to induce the generation of protective T cells is due not to the lack of a central T-cell epitope(s) but to the lack of ability to induce the production of endogenous cytokine during the early stage of immunization; the results also suggest that an appropriate use of LLO at least in an animal model may be effective in the induction of antigen-specific Th1-dependent protective immunity to various kinds of intracellular parasitic bacteria.
Figures








References
-
- Berche P, Gaillard J, Sansonetti P J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. - PubMed
-
- Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a hemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature (London) 1990;345:175–176. - PubMed
-
- Colette L B, James P B, Henry A F, Peter A V. Kinetics of phagocytosis and phagosome-lysosome fusion in hamster lung and peritoneal macrophages. J Leukoc Biol. 1991;50:229–239. - PubMed
-
- Darji A, Chakraborty T, Wehland J, Weiss S. TAP-dependent major histocompatibility complex class I presentation of soluble protein using listeriolysin. Eur J Immunol. 1997;27:1353–1359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

