Use of an isogenic mutant constructed in Moraxella catarrhalis To identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6
- PMID: 9916077
- PMCID: PMC96373
- DOI: 10.1128/IAI.67.2.681-687.1999
Use of an isogenic mutant constructed in Moraxella catarrhalis To identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6
Abstract
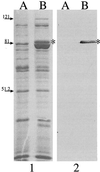
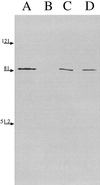
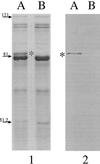
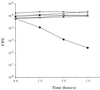
Moraxella catarrhalis-induced otitis media continues to be a significant cause of infection in young children, prompting increased efforts at identifying effective vaccine antigens. We have previously demonstrated that M. catarrhalis expresses specific outer membrane proteins (OMPs) in response to iron limitation and that this organism can utilize transferrin and lactoferrin for in vitro growth. One of these proteins, which binds human transferrin, is OMP B1. As the human host presents a naturally iron-limited environment, proteins, like OMP B1, which are expressed in response to this nutritional stress are potential vaccine antigens. In this study, we have developed monoclonal antibody (MAb) 11C6, which reacts to a surface-exposed epitope of OMP B1 expressed by M. catarrhalis 7169. This antibody was used to clone ompB1, and sequence analysis suggested that OMP B1 is the M. catarrhalis homologue to the transferrin binding protein B described for pathogenic Neisseriaceae, Haemophilus influenzae, Actinobacillus pleuropneumoniae, and M. catarrhalis. Expression of recombinant OMP B1 on the surface of Escherichia coli confers transferrin binding activity, confirming that this protein is likely involved in iron acquisition. In addition, ompB1 was used to construct an isogenic mutant in M. catarrhalis 7169. This mutant, termed 7169b12, was used as the control in bactericidal assays designed to determine if OMP B1 elicits protective antibodies. In the presence of MAb 11C6 and human complement, wild-type 7169 demonstrated a 99% decline in viability, whereas the ompB1 isogenic mutant was resistant to this bactericidal activity. Further analysis with MAb 11C6 revealed the presence of this OMP B1 epitope on 31% of the clinical isolates tested. These data suggest that OMP B1 is a potential vaccine antigen against M. catarrhalis infections.
Figures

Similar articles
-
Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis.Infect Immun. 1999 Sep;67(9):4578-85. doi: 10.1128/IAI.67.9.4578-4585.1999. Infect Immun. 1999. PMID: 10456903 Free PMC article.
-
Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin.Infect Immun. 1996 Sep;64(9):3920-4. doi: 10.1128/iai.64.9.3920-3924.1996. Infect Immun. 1996. PMID: 8751951 Free PMC article.
-
The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen.Infect Immun. 1998 Sep;66(9):4183-92. doi: 10.1128/IAI.66.9.4183-4192.1998. Infect Immun. 1998. PMID: 9712766 Free PMC article.
-
Vaccines for Moraxella catarrhalis.Vaccine. 2000 Dec 8;19 Suppl 1:S101-7. doi: 10.1016/s0264-410x(00)00287-5. Vaccine. 2000. PMID: 11163472 Review.
-
Complement evasion by the human respiratory tract pathogens Haemophilus influenzae and Moraxella catarrhalis.FEBS Lett. 2020 Aug;594(16):2586-2597. doi: 10.1002/1873-3468.13758. Epub 2020 Mar 9. FEBS Lett. 2020. PMID: 32053211 Review.
Cited by
-
Respiratory syncytial virus promotes Moraxella catarrhalis-induced ascending experimental otitis media.PLoS One. 2012;7(6):e40088. doi: 10.1371/journal.pone.0040088. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768228 Free PMC article.
-
Characterization of a cluster of three glycosyltransferase enzymes essential for Moraxella catarrhalis lipooligosaccharide assembly.J Bacteriol. 2005 May;187(9):2939-47. doi: 10.1128/JB.187.9.2939-2947.2005. J Bacteriol. 2005. PMID: 15838019 Free PMC article.
-
The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells.Infect Immun. 2004 Apr;72(4):1906-13. doi: 10.1128/IAI.72.4.1906-1913.2004. Infect Immun. 2004. PMID: 15039309 Free PMC article.
-
Inactivation of the Moraxella catarrhalis superoxide dismutase SodA induces constitutive expression of iron-repressible outer membrane proteins.Infect Immun. 2002 Apr;70(4):1889-95. doi: 10.1128/IAI.70.4.1889-1895.2002. Infect Immun. 2002. PMID: 11895952 Free PMC article.
-
Lipooligosaccharide P(k) (Galalpha1-4Galbeta1-4Glc) epitope of moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum.Infect Immun. 2000 Sep;68(9):5261-8. doi: 10.1128/IAI.68.9.5261-5268.2000. Infect Immun. 2000. PMID: 10948153 Free PMC article.
References
-
- Ala’Aldeen D A. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol. 1996;44:237–243. - PubMed
-
- Ala’Aldeen D A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. - PubMed
-
- Bluestone C D. Modern management of otitis media. Pediatr Clin N Am. 1989;36:1371–1387. - PubMed
-
- Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

