Expression and distribution of leptospiral outer membrane components during renal infection of hamsters
- PMID: 9916100
- PMCID: PMC96396
- DOI: 10.1128/IAI.67.2.853-861.1999
Expression and distribution of leptospiral outer membrane components during renal infection of hamsters
Abstract
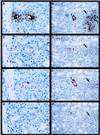
The outer membrane of pathogenic Leptospira species grown in culture media contains lipopolysaccharide (LPS), a porin (OmpL1), and several lipoproteins, including LipL36 and LipL41. The purpose of this study was to characterize the expression and distribution of these outer membrane antigens during renal infection. Hamsters were challenged with host-derived Leptospira kirschneri to generate sera which contained antibodies to antigens expressed in vivo. Immunoblotting performed with sera from animals challenged with these host-derived organisms demonstrated reactivity with OmpL1, LipL41, and several other proteins but not with LipL36. Although LipL36 is a prominent outer membrane antigen of cultivated L. kirschneri, its expression also could not be detected in infected hamster kidney tissue by immunohistochemistry, indicating that expression of this protein is down-regulated in vivo. In contrast, LPS, OmpL1, and LipL41 were demonstrated on organisms colonizing the lumen of proximal convoluted renal tubules at both 10 and 28 days postinfection. Tubular epithelial cells around the luminal colonies had fine granular cytoplasmic LPS. When the cellular inflammatory response was present in the renal interstitium at 28 days postinfection, LPS and OmpL1 were also detectable within interstitial phagocytes. These data establish that outer membrane components expressed during infection have roles in the induction and persistence of leptospiral interstitial nephritis.
Figures



References
-
- Alexander A D, Lessel E F, Evans L B, Franck E, Green S S. Preservation of leptospiras by liquid-nitrogen refrigeration. Int J Syst Bacteriol. 1972;22:165–169.
-
- Alves V A, Gayotto L C, Yasuda P H, Wakamatsu A, Kanamura C T, De Brito T. Leptospiral antigens (L. interrogans serogroup icterohaemorrhagiae) in the kidney of experimentally infected guinea pigs and their relation to the pathogenesis of the renal injury. Exp Pathol. 1991;42:81–93. - PubMed
-
- Alves V A F, Vianna M R, Yasuda P H, DeBrito T. Detection of leptospiral antigen in the human liver and kidney using an immunoperoxidase staining procedure. J Pathol. 1987;151:125–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

