In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen
- PMID: 9916105
- PMCID: PMC96401
- DOI: 10.1128/IAI.67.2.891-898.1999
In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen
Abstract
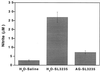
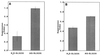
Our laboratory has previously shown that after immunization with a strain of Salmonella typhimurium, SL3235, made avirulent by a blockage in the pathway of aromatic synthesis, murine splenocytes were profoundly suppressed in their capacity to mount an in vitro antibody plaque-forming cell (PFC) response to sheep erythrocytes. Evidence indicated that suppression was mediated by nitric oxide (NO), since the in vitro addition of NG-monomethyl-L-arginine blocked suppression. The present studies examined the effect of blocking NO production on Salmonella-induced immunosuppression by in vivo administration of aminoguanidine hemisulfate (AG). AG was administered to C3HeB/FeJ mice in their drinking water (2.5% solution) for 7 days prior to intraperitoneal inoculation with SL3235. AG treatment inhibited the increase in nitrate and nitrite levels in plasma and nitrite levels in the spleen seen in immunized mice. Importantly, AG treatment completely blocked suppression of the splenic PFC response and markedly attenuated the suppression of the response to concanavalin A in immunized mice, providing further evidence that Salmonella-induced immunosuppression is mediated by NO. AG treatment also alleviated the majority of the splenomegaly associated with SL3235 inoculation, which correlated with a blockage of influx of neutrophils and macrophages into spleens, as assessed by flow cytometry. AG treatment unexpectedly resulted in 90% mortality in mice injected with the highly attenuated vaccine strain of Salmonella, SL3235. Increased mortality in AG-treated mice correlated with inability to clear organisms from the spleen by day 15 postinoculation and with persistent bacteremia, compared with control mice. Collectively, these in vivo results underscore the dual biological consequences of NO production following Salmonella infection, with NO being necessary for host defense, but also having the potentially adverse effect of immunosuppression. A unifying hypothesis to explain how these seemingly paradoxical effects could both result from NO production is presented.
Figures




Similar articles
-
Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression.Microb Pathog. 1992 Apr;12(4):267-78. doi: 10.1016/0882-4010(92)90045-p. Microb Pathog. 1992. PMID: 1630297
-
Attenuated Salmonella vaccine-induced suppression of murine spleen cell responses to mitogen is mediated by macrophage nitric oxide: quantitative aspects.Infect Immun. 1996 Sep;64(9):3786-92. doi: 10.1128/iai.64.9.3786-3792.1996. Infect Immun. 1996. PMID: 8751930 Free PMC article.
-
Nitric oxide mediates immunosuppression induced by Listeria monocytogenes infection: quantitative studies.Microb Pathog. 1998 Nov;25(5):267-77. doi: 10.1006/mpat.1998.0238. Microb Pathog. 1998. PMID: 9878455
-
Macrophage nitric oxide mediates immunosuppression in infectious inflammation.Immunobiology. 1994 Oct;191(4-5):493-502. doi: 10.1016/S0171-2985(11)80455-9. Immunobiology. 1994. PMID: 7713563 Review.
-
Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule.Microbes Infect. 2001 Nov-Dec;3(14-15):1223-31. doi: 10.1016/s1286-4579(01)01482-4. Microbes Infect. 2001. PMID: 11755410 Review.
Cited by
-
Gonococci cause immunosuppression by engaging a coinhibitory receptor on T lymphocytes.Nat Immunol. 2002 Mar;3(3):210-1. doi: 10.1038/ni0302-210. Nat Immunol. 2002. PMID: 11875456 Free PMC article.
-
Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles.Cell Adh Migr. 2013 Mar-Apr;7(2):174-86. doi: 10.4161/cam.23130. Epub 2013 Jan 3. Cell Adh Migr. 2013. PMID: 23287580 Free PMC article. Review.
-
Igh-6(-/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens.Infect Immun. 2000 Jan;68(1):46-53. doi: 10.1128/IAI.68.1.46-53.2000. Infect Immun. 2000. PMID: 10603367 Free PMC article.
-
Characterization of an outer membrane protein of Salmonella enterica serovar typhimurium that confers protection against typhoid.Clin Vaccine Immunol. 2008 Sep;15(9):1461-71. doi: 10.1128/CVI.00093-08. Epub 2008 Jul 23. Clin Vaccine Immunol. 2008. PMID: 18650399 Free PMC article.
-
Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities.Infect Immun. 2002 Jun;70(6):3130-42. doi: 10.1128/IAI.70.6.3130-3142.2002. Infect Immun. 2002. PMID: 12011007 Free PMC article.
References
-
- Adams L B, Franzblau S G, Vavrin Z, Hibbs J B, Jr, Krahenbuhl J L. l-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991;147:1642–1646. - PubMed
-
- Al-Ramadi B K, Brodkin M A, Mosser D M, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–2746. - PubMed
-
- Al-Ramadi B K, Greene J M, Meissler J J, Jr, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression. Microb Pathog. 1992;12:267–278. - PubMed
-
- Al-Ramadi B K, Meissler J J, Jr, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22:2249–2254. - PubMed
-
- Anthony L S D, Morrissey P J, Nano F E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-γ-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J Immunol. 1992;148:1829–1834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

