Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions
- PMID: 9916108
- PMCID: PMC96404
- DOI: 10.1128/IAI.67.2.914-920.1999
Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions
Abstract
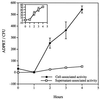
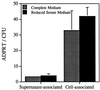
This study was initiated to characterize the regulation and secretion of ExoS by Pseudomonas aeruginosa during contact with eukaryotic cells. The production of ExoS was monitored by a sensitive ADP-ribosyltransferase activity assay, and specific activities were calculated for supernatant and cell-associated fractions. Time course analysis indicated that ExoS was produced after a lag period, suggesting that induction of the regulon is necessary for the expression of detectable amounts of enzyme activity. Under tissue culture growth conditions, ExoS was induced when P. aeruginosa was in contact with Chinese hamster ovary (CHO) cells or after growth in tissue culture medium with serum. The serum induction of ExoS appeared to result in generalized type III secretion, while induction by contact with CHO cells appeared to result in polarized type III secretion. Mutants in the type III secretory system that express a null phenotype for ExoS production in bacteriological medium produced but did not secrete the enzyme when P. aeruginosa was grown under inducing conditions in tissue culture medium. These results suggest that both induction and secretion of ExoS may differ when the bacteria are exposed to different growth environments. The putative type III translocation proteins and secretion apparatus of P. aeruginosa were required for translocation of bacterial factors that mediate changes in CHO cell morphology during infection.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

