Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal
- PMID: 9916127
- PMCID: PMC407882
- DOI: 10.1172/JCI5028
Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal
Abstract
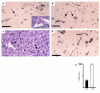
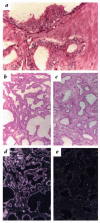
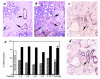
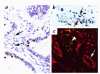
Features that distinguish tumor vasculatures from normal blood vessels are sought to enable the destruction of preformed tumor vessels. We show that blood vessels in both a xenografted tumor and primary human tumors contain a sizable fraction of immature blood vessels that have not yet recruited periendothelial cells. These immature vessels are selectively obliterated as a consequence of vascular endothelial growth factor (VEGF) withdrawal. In a xenografted glioma, the selective vulnerability of immature vessels to VEGF loss was demonstrated by downregulating VEGF transgene expression using a tetracycline-regulated expression system. In human prostate cancer, the constitutive production of VEGF by the glandular epithelium was suppressed as a consequence of androgen-ablation therapy. VEGF loss led, in turn, to selective apoptosis of endothelial cells in vessels devoid of periendothelial cells. These results suggest that the unique dependence on VEGF of blood vessels lacking periendothelial cells can be exploited to reduce an existing tumor vasculature.
Figures

Comment in
-
Blood vessel maturation: vascular development comes of age.J Clin Invest. 1999 Jan;103(2):157-8. doi: 10.1172/JCI6127. J Clin Invest. 1999. PMID: 9916126 Free PMC article. No abstract available.
References
-
- Folkman, J. 1997. Cancer: principles and practice of oncology. Lippincott-Raven Publishers. Philadelphia, PA. 3075–3085.
-
- Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. - PubMed
-
- Millauer B, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. - PubMed
-
- Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996;56:4032–4039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

