TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development
- PMID: 9916131
- PMCID: PMC407876
- DOI: 10.1172/JCI3523
TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development
Abstract
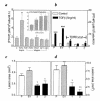
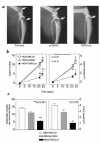
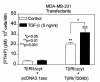
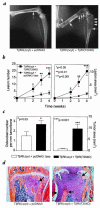
Breast cancer frequently metastasizes to the skeleton, and the associated bone destruction is mediated by the osteoclast. Growth factors, including transforming growth factor-beta (TGF-beta), released from bone matrix by the action of osteoclasts, may foster metastatic growth. Because TGF-beta inhibits growth of epithelial cells, and carcinoma cells are often defective in TGF-beta responses, any role of TGF-beta in metastasis is likely to be mediated by effects on the surrounding normal tissue. However, we present evidence that TGF-beta promotes breast cancer metastasis by acting directly on the tumor cells. Expression of a dominant-negative mutant (TbetaRIIDeltacyt) of the TGF-beta type II receptor rendered the human breast cancer cell line MDA-MB-231 unresponsive to TGF-beta. In a murine model of bone metastases, expression of TbetaRIIDeltacyt by MDA-MB-231 resulted in less bone destruction, less tumor with fewer associated osteoclasts, and prolonged survival compared with controls. Reversal of the dominant-negative signaling blockade by expression of a constitutively active TGF-beta type I receptor in the breast cancer cells increased tumor production of parathyroid hormone-related protein (PTHrP), enhanced osteolytic bone metastasis, and decreased survival. Transfection of MDA-MB-231 cells that expressed the dominant-negative TbetaRIIDeltacyt with the cDNA for PTHrP resulted in constitutive tumor PTHrP production and accelerated bone metastases. These data demonstrate an important role for TGF-beta in the development of breast cancer metastasis to bone, via the TGF-beta receptor-mediated signaling pathway in tumor cells, and suggest that the bone destruction is mediated by PTHrP.
Figures
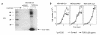

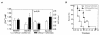

References
-
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–572. - PubMed
-
- Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. J Biol Chem. 1986;261:12665–12674. - PubMed
-
- Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR. Scanning electron microscopy in bone pathology: review of methods. Potential and applications. Scanning Electron Microsc. 1986;4:1537–1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

