Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis
- PMID: 9916919
- PMCID: PMC1853427
- DOI: 10.1016/S0002-9440(10)65251-0
Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis
Abstract
Microdissection of routinely stained or unstained frozen sections has been used successfully to obtain purified cell populations for the analysis of cell-specific gene expression patterns in primary tissues with a complex mixture of cell types. However, the precision and usefulness of microdissection is frequently limited by the difficulty to identify different cell types and structures by morphology alone. We therefore developed a rapid immunostaining procedure for frozen sections followed by laser capture microdissection (LCM) and RNA extraction, which allows targeted mRNA analysis of immunophenotypically defined cell populations. After fixation, frozen sections are immunostained under RNAse-free conditions using a rapid three-step streptavidin-biotin technique, dehydrated and immediately subjected to LCM. RNA is extracted from captured tissue, DNAse I treated, and reverse transcribed. Acetone-, methanol-, or ethanol/acetone-fixed sections give excellent immunostaining after 12 to 25 minutes total processing time. Specificity, precision, and speed of microdissection is markedly increased due to improved identification of desired (or undesired) cell types. The mRNA recovered from immunostained tissue is of high quality. Single-step PCR is able to amplify fragments of more than 600 bp from both housekeeping genes such as beta-actin as well as cell-specific messages such as CD4 or CD19, using cDNA derived from less than 500 immunostained, microdissected cells. Immuno-LCM allows specific mRNA analysis of cell populations isolated according to their immunophenotype or expression of function-related antigens and significantly expands our ability to investigate gene expression in heterogeneous tissues.
Figures
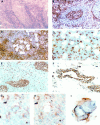


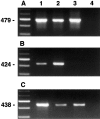
References
-
- Whetsell L, Maw G, Nadon N, Ringer PD, Schaefer FV: Polymerase chain reaction microanalysis of tumors from stained histological slides. Oncogene 1992, 7:2355-2361 - PubMed
-
- Going JJ, Lamb RF: Practical histological microdissection for PCR analysis. J Pathol 1996, 179:121-124 - PubMed
-
- Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui R, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA: Laser capture microdissection. Science 1996, 274:998-1001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

