Hereditary amyloid cardiomyopathy caused by a variant apolipoprotein A1
- PMID: 9916936
- PMCID: PMC1853430
- DOI: 10.1016/S0002-9440(10)65268-6
Hereditary amyloid cardiomyopathy caused by a variant apolipoprotein A1
Abstract
Autosomal dominant hereditary amyloidosis with a unique cutaneous and cardiac presentation and death from heart failure by the sixth or seventh decade was found to be associated with a previously unreported point mutation (thymine to cytosine, nt 1389) in exon 4 of the apolipoprotein A1 (apoA1) gene. The predicted substitution of proline for leucine at amino acid position 90 was confirmed by structural analysis of amyloid protein isolated from cardiac deposits of amyloid. The subunit protein is composed exclusively of NH2-terminal fragments of the variant apoA1 with the longest ending at residue 94 in the wild-type sequence. Amyloid fibrils derived from four previously described apoA1 variants are composed of similar fragments with carboxyl-terminal heterogeneity, but contrary to those variants, which all carry one extra positive charge, the substitution Leu90Pro does not result in any charge modification. It is unlikely, therefore, that amyloid fibril formation is related to change of charge for a specific residue of the precursor protein. This is in agreement with studies on transthyretin amyloidosis in which no unifying factor such as change of charge for amino acid residues has been noted.
Figures

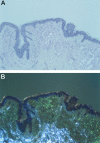


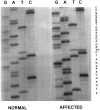
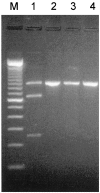
Comment in
-
A novel amyloidogenic variant of apolipoprotein AI: implications for a conformational change leading to cardiomyopathy.Am J Pathol. 1999 Jan;154(1):11-4. doi: 10.1016/S0002-9440(10)65244-3. Am J Pathol. 1999. PMID: 9916912 Free PMC article. Review. No abstract available.
References
-
- Benson MD: Amyloidosis. ed 7 Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic Basis of Inherited Disease, 1995, :pp 4159-4191 McGraw-Hill, New York
-
- Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James R, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J: Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349:704-706 - PubMed
-
- Murrell J, Farlow M, Ghetti B, Benson MD: A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991, 254:97-99 - PubMed
-
- Benson MD, Uemichi T: Transthyretin amyloidosis. Amyloid 1996, 3:44-51
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

