Ca2+-induced deprotonation of peptide hormones inside secretory vesicles in preparation for release
- PMID: 9920653
- PMCID: PMC6782154
- DOI: 10.1523/JNEUROSCI.19-03-00900.1999
Ca2+-induced deprotonation of peptide hormones inside secretory vesicles in preparation for release
Abstract
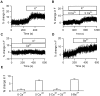
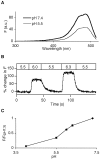
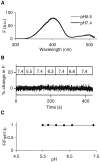
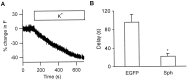
The acidic environment inside secretory vesicles ensures that neuropeptides and peptide hormones are packaged in a concentrated condensed form. Although this is optimal for storage, decondensation limits release. Thus, it would be advantageous to alter the physical state of peptides in preparation for exocytosis. Here, we report that depolarization of the plasma membrane rapidly increases enhanced green fluorescent protein (EGFP)-tagged hormone fluorescence inside secretory vesicles. This effect requires Ca2+ influx and persists when exocytosis is inhibited by N-ethylmaleimide. Peptide deprotonation appears to produce this response, because it is not seen when the vesicle pH gradient is collapsed or when a pH-insensitive GFP variant is used. These data demonstrate that Ca2+ evokes alkalinization of the inside of secretory vesicles before exocytosis. Thus, Ca2+ influx into the cytoplasm alters the physical state of intravesicular contents in preparation for release.
Figures



Similar articles
-
Neuronal peptide release is limited by secretory granule mobility.Neuron. 1997 Nov;19(5):1095-102. doi: 10.1016/s0896-6273(00)80400-6. Neuron. 1997. PMID: 9390522
-
Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis.J Neurosci. 2003 Nov 19;23(33):10531-9. doi: 10.1523/JNEUROSCI.23-33-10531.2003. J Neurosci. 2003. PMID: 14627637 Free PMC article.
-
A new green fluorescent protein construct for localizing and quantifying peptide release.Ann N Y Acad Sci. 2002 Oct;971:627-33. doi: 10.1111/j.1749-6632.2002.tb04541.x. Ann N Y Acad Sci. 2002. PMID: 12438197
-
Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles.Pflugers Arch. 2004 Jul;448(4):347-62. doi: 10.1007/s00424-004-1247-8. Epub 2004 Mar 2. Pflugers Arch. 2004. PMID: 14997396 Review.
-
Insulin secretion by 'kiss-and-run' exocytosis in clonal pancreatic islet beta-cells.Biochem Soc Trans. 2003 Aug;31(Pt 4):833-6. doi: 10.1042/bst0310833. Biochem Soc Trans. 2003. PMID: 12887316 Review.
Cited by
-
Matrix-dependent local retention of secretory vesicle cargo in cortical neurons.J Neurosci. 2009 Jan 7;29(1):23-37. doi: 10.1523/JNEUROSCI.3931-08.2009. J Neurosci. 2009. PMID: 19129381 Free PMC article.
-
Expression of familial Alzheimer disease presenilin 1 gene attenuates vesicle traffic and reduces peptide secretion in cultured astrocytes devoid of pathologic tissue environment.Glia. 2016 Feb;64(2):317-29. doi: 10.1002/glia.22931. Epub 2015 Oct 14. Glia. 2016. PMID: 26462451 Free PMC article.
-
Unexpected mobility variation among individual secretory vesicles produces an apparent refractory neuropeptide pool.Biophys J. 2003 Jun;84(6):4127-34. doi: 10.1016/S0006-3495(03)75137-6. Biophys J. 2003. PMID: 12770915 Free PMC article.
-
Activity-dependent vesicular monoamine transporter-mediated depletion of the nucleus supports somatic release by serotonin neurons.J Neurosci. 2009 Dec 16;29(50):15878-87. doi: 10.1523/JNEUROSCI.4210-09.2009. J Neurosci. 2009. PMID: 20016104 Free PMC article.
-
Nerve growth factor-induced differentiation changes the cellular organization of regulated Peptide release by PC12 cells.J Neurosci. 2002 May 15;22(10):3890-7. doi: 10.1523/JNEUROSCI.22-10-03890.2002. J Neurosci. 2002. PMID: 12019308 Free PMC article.
References
-
- Arrandale JM, Dannies PS. Inhibition of rat prolactin (PRL) storage by coexpression of human PRL. Mol Endocrinol. 1994;8:1083–1089. - PubMed
-
- Artalejo CR, Elhamdani A, Palfrey HC. Secretion: dense-core granules can kiss-and-run too. Curr Biol. 1998;8:R62–R65. - PubMed
-
- Aspinwall CA, Brooks SA, Kennedy RT, Lakey JRT. Effects of intravesicular H+ and extracellular H+ and Zn2+ on insulin secretion in pancreatic beta cells. J Biol Chem. 1997;272:31308–31314. - PubMed
-
- Burke NV, Han W, Li D, Takimoto K, Watkins SC, Levitan ES. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
