Prostaglandin E2 stimulates amyloid precursor protein gene expression: inhibition by immunosuppressants
- PMID: 9920657
- PMCID: PMC6782137
- DOI: 10.1523/JNEUROSCI.19-03-00940.1999
Prostaglandin E2 stimulates amyloid precursor protein gene expression: inhibition by immunosuppressants
Abstract
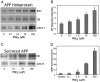
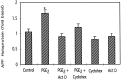
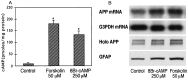
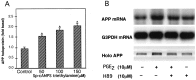
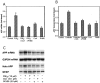
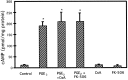
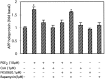
Amyloid plaques that accumulate in the brains of patients with Alzheimer's disease (AD) are primarily composed of aggregates of amyloid peptides that are derived from the amyloid precursor protein (APP). Overexpression of APP in cell cultures increases the formation of amyloidogenic peptides and causes neurodegeneration and cognitive dysfunction in transgenic mice. We now report that activation of prostaglandin E2 (PGE2) receptors increases cAMP formation and stimulates overexpression of APP mRNA and holoprotein in primary cultures of cortical astrocytes. Levels of glial fibrillary acidic protein were also increased by PGE2 treatment, suggesting that these cultured astrocytes resemble reactive astrocytes found in vivo. The stimulation by PGE2 of APP synthesis was mimicked or blocked by activators or inhibitors, respectively, of protein kinase A. Actinomycin D or cycloheximide also inhibited the increase in APP holoprotein stimulated by PGE2. Treatment of astrocytes with 8-Bromo-cAMP or forskolin for 24 hr also stimulated APP overexpression in cultured astrocytes. The immunosuppressants cyclosporin A and FK-506 inhibited the increase in APP mRNA and holoprotein levels caused by PGE2 or by other treatments that elevated cellular cAMP levels; the inhibitory effect of FK-506 but not of cyclosporin A was attenuated by rapamycin. These results suggest that prostaglandins produced by brain injury or inflammation can activate APP transcription in astrocytes and that immunosuppressants may be used to prevent APP overexpression and possibly the pathophysiological processes underlying AD.
Figures
Similar articles
-
Stimulation of amyloid precursor protein synthesis by adrenergic receptors coupled to cAMP formation.Proc Natl Acad Sci U S A. 1997 May 13;94(10):5422-6. doi: 10.1073/pnas.94.10.5422. Proc Natl Acad Sci U S A. 1997. PMID: 9144253 Free PMC article.
-
Regulation of APP synthesis and secretion by neuroimmunophilin ligands and cyclooxygenase inhibitors.Ann N Y Acad Sci. 2000;920:261-8. doi: 10.1111/j.1749-6632.2000.tb06934.x. Ann N Y Acad Sci. 2000. PMID: 11193162 Review.
-
Intracellular and cell-surface distribution of amyloid precursor protein in cortical astrocytes.Brain Res Bull. 1999 Sep 1;50(1):27-32. doi: 10.1016/s0361-9230(99)00084-2. Brain Res Bull. 1999. PMID: 10507468
-
Prostaglandin E2 regulates amyloid precursor protein expression via the EP2 receptor in cultured rat microglia.Neurosci Lett. 2004 May 20;362(2):127-30. doi: 10.1016/j.neulet.2004.03.013. Neurosci Lett. 2004. PMID: 15193769
-
Overexpression of amyloid precursor protein is associated with degeneration, decreased viability, and increased damage caused by neurotoxins (prostaglandins A1 and E2, hydrogen peroxide, and nitric oxide) in differentiated neuroblastoma cells.J Neurosci Res. 2003 Oct 1;74(1):148-59. doi: 10.1002/jnr.10726. J Neurosci Res. 2003. PMID: 13130517
Cited by
-
Role of prostaglandins in neuroinflammatory and neurodegenerative diseases.Mediators Inflamm. 2012;2012:946813. doi: 10.1155/2012/946813. Epub 2012 Jun 18. Mediators Inflamm. 2012. PMID: 22778499 Free PMC article. Review.
-
Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology.Gene Expr. 2002;10(5-6):271-8. doi: 10.3727/000000002783992352. Gene Expr. 2002. PMID: 12450219 Free PMC article.
-
Calcium Ions Stimulate the Hyperphosphorylation of Tau by Activating Microsomal Prostaglandin E Synthase 1.Front Aging Neurosci. 2019 May 9;11:108. doi: 10.3389/fnagi.2019.00108. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31143112 Free PMC article.
-
P2Y2 nucleotide receptor-mediated responses in brain cells.Mol Neurobiol. 2010 Jun;41(2-3):356-66. doi: 10.1007/s12035-010-8115-7. Epub 2010 Apr 13. Mol Neurobiol. 2010. PMID: 20387013 Free PMC article. Review.
-
Targeting Tumor Necrosis Factor Alpha for Alzheimer's Disease.Curr Alzheimer Res. 2017;14(4):412-425. doi: 10.2174/1567205013666160930110551. Curr Alzheimer Res. 2017. PMID: 27697064 Free PMC article. Review.
References
-
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr Psychol-Gerichtl Med. 1907;64:146–148.
-
- Banati RB, Gehrmann J, Wieβner C, Hossman K-A, Kreutzberg GW. Glial expression of the β-amyloid precursor protein (APP) in global ischemia. J Cereb Blood Flow Metab. 1995;12:257–269. - PubMed
-
- Bourbonniére M, Shekarabi M, Nalbantoglu J. Enhanced expression of amyloid precursor protein in response to dibutyryl cyclic AMP is not mediated by the transcription factor AP-2. J Neurochem. 1997;68:909–916. - PubMed
-
- Brun A, Liu X, Erikson C. Synapse loss and gliosis in the molecular layer of cerebral cortex in Alzheimer’s disease and in frontal lobe degeneration. Neurodegeneration. 1995;4:171–177. - PubMed
-
- Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer R. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
