Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor
- PMID: 9920658
- PMCID: PMC6782144
- DOI: 10.1523/JNEUROSCI.19-03-00948.1999
Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor
Abstract
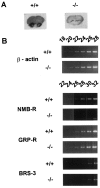
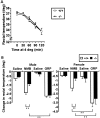
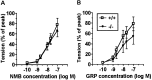
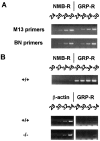
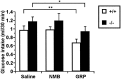
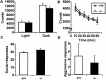
The neuromedin B-preferring receptor (NMB-R) is one of the members of the bombesin (BN)-like peptide receptor subfamily in mammals. Previously, we have generated and characterized mice with targeted disruption of the two other BN-like peptide receptors, bombesin receptor subtype-3 (BRS-3) and gastrin-releasing peptide-preferring receptor (GRP-R). Here we describe the generation and analysis of NMB-R-deficient mice to investigate how NMB-R differs from BRS-3 and GRP-R. Compensation for NMB-R deficiency by overexpression of GRP-R and/or BRS-3 was not detected. Although the hypothermic effect of NMB was reduced by 50% in NMB-R-deficient mice, the effect of GRP infusion was comparable to the wild-type mice. In contrast, fundic smooth muscle contraction on stimulation with NMB or GRP was normal in NMB-R-deficient mice. Administration of GRP but not NMB suppressed glucose intake in both normal and NMB-R-deficient mice. These results suggest that the NMB-R has an essential role in thermoregulation, but not for smooth muscle contraction of the fundus or for the suppression of feeding behavior. In addition, the behavioral phenotypes of GRP-R-deficient mice were not observed in NMB-R-deficient mice. These data show that the functions of NMB-R and GRP-R are distinct, with only partial overlap.
Figures

Similar articles
-
Disruptions in feeding and body weight control in gastrin-releasing peptide receptor deficient mice.J Endocrinol. 2002 Aug;174(2):273-81. doi: 10.1677/joe.0.1740273. J Endocrinol. 2002. PMID: 12176666
-
Role of different bombesin receptor subtypes mediating contractile activity in cat upper gastrointestinal tract.Peptides. 1998;19(3):549-56. doi: 10.1016/s0196-9781(97)00467-1. Peptides. 1998. PMID: 9533644
-
Leptin resistance and enhancement of feeding facilitation by melanin-concentrating hormone in mice lacking bombesin receptor subtype-3.Diabetes. 2004 Mar;53(3):570-6. doi: 10.2337/diabetes.53.3.570. Diabetes. 2004. PMID: 14988239
-
Bombesin-like peptides: studies on food intake and social behaviour with receptor knock-out mice.Ann Med. 2000 Nov;32(8):519-29. doi: 10.3109/07853890008998831. Ann Med. 2000. PMID: 11127929 Review.
-
Development and function of bombesin-like peptides and their receptors.Int J Dev Biol. 2005;49(2-3):293-300. doi: 10.1387/ijdb.041954ho. Int J Dev Biol. 2005. PMID: 15906244 Review.
Cited by
-
Gastrin-releasing peptide receptor mediates activation of the epidermal growth factor receptor in lung cancer cells.Neoplasia. 2005 Apr;7(4):426-31. doi: 10.1593/neo.04454. Neoplasia. 2005. PMID: 15967120 Free PMC article.
-
Oxygen, gastrin-releasing Peptide, and pediatric lung disease: life in the balance.Front Pediatr. 2014 Jul 18;2:72. doi: 10.3389/fped.2014.00072. eCollection 2014. Front Pediatr. 2014. PMID: 25101250 Free PMC article. Review.
-
Cross-inhibition of NMBR and GRPR signaling maintains normal histaminergic itch transmission.J Neurosci. 2014 Sep 10;34(37):12402-14. doi: 10.1523/JNEUROSCI.1709-14.2014. J Neurosci. 2014. PMID: 25209280 Free PMC article.
-
A nociceptive signaling role for neuromedin B.J Neurosci. 2012 Jun 20;32(25):8686-95. doi: 10.1523/JNEUROSCI.1533-12.2012. J Neurosci. 2012. PMID: 22723708 Free PMC article.
-
Insights into bombesin receptors and ligands: Highlighting recent advances.Peptides. 2015 Oct;72:128-44. doi: 10.1016/j.peptides.2015.04.026. Epub 2015 May 11. Peptides. 2015. PMID: 25976083 Free PMC article. Review.
References
-
- Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the european amphibians Bombina and Alytes. Experientia. 1971;27:166–167. - PubMed
-
- Corjay MH, Dobrzanski DJ, Way JM, Viallet J, Shapira H, Worland P, Sausville EA, Battey JE. Two distinct bombesin receptor subtypes are expressed and functional in human lung carcinoma cells. J Biol Chem. 1991;266:18771–18779. - PubMed
-
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. A novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268:5979–5984. - PubMed
-
- Gorbulev V, Akhundova A, Buchner H, Fahrenholz F. Molecular cloning of a new bombesin receptor subtype expressed in uterus during pregnancy. Eur J Biochem. 1992;208:405–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
