Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms
- PMID: 9920673
- PMCID: PMC6782139
- DOI: 10.1523/JNEUROSCI.19-03-01115.1999
Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms
Abstract
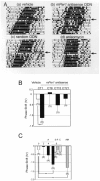
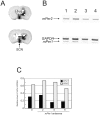
mPer1, a mouse gene, is a homolog of the Drosophila clock gene period and has been shown to be closely associated with the light-induced resetting of a mammalian circadian clock. To investigate whether the rapid induction of mPer1 after light exposure is necessary for light-induced phase shifting, we injected an antisense phosphotioate oligonucleotide (ODN) to mPer1 mRNA into the cerebral ventricle. Light-induced phase delay of locomotor activity at CT16 was significantly inhibited when the mice were pretreated with mPer1 antisense ODN 1 hr before light exposure. mPer1 sense ODN or random ODN treatment had little effect on phase delay induced by light pulses. In addition, glutamate-induced phase delay of suprachiasmatic nucleus (SCN) firing rhythm was attenuated by pretreatment with mPer1 antisense ODN, but not by random ODN. The present results demonstrate that induction of mPer1 mRNA is required for light- or glutamate-induced phase shifting, suggesting that the acute induction of mPer1 mRNA in the SCN after light exposure is involved in light-induced phase shifting of the overt rhythm.
Figures


References
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mPer1 and mPer2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990;523:117–120. - PubMed
-
- Daan S, Pittendrigh C. A functional analysis of circadian pacemakers in nocturnal rodents II. The variability of phase response curves. J Comp Physiol [A] 1976;106:253–266.
-
- de Vries MJ, Nunes Cardozo B, van der Want J, de Wolf A, Meijer JH. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain Res. 1993;612:231–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
