Fibrillar array in the cell wall of a gliding filamentous cyanobacterium
- PMID: 9922252
- PMCID: PMC93455
- DOI: 10.1128/JB.181.3.884-892.1999
Fibrillar array in the cell wall of a gliding filamentous cyanobacterium
Abstract
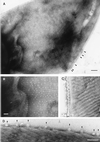
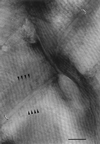
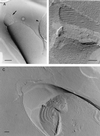
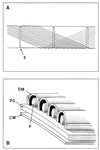
The cell walls of a number of filamentous, gliding cyanobacteria of the genus Oscillatoria were examined by transmission electron microscopy of ultrathin sections, of freeze-etched replicas, and of whole cells crushed between glass slides and negatively stained. All three techniques revealed the presence of a highly ordered array of parallel fibrils, seen in transverse sections to be situated between the peptidoglycan and the outer membrane. Approximately 200 individual fibrils, each 25 to 30 nm in width, form a parallel, helical array that completely surrounds each cyanobacterial filament, running at an angle of 25 to 30 degrees to its long axis. This highly regular arrangement of the fibrillar layer may imply some underlying symmetry responsible for its organization. A possible source of such symmetry would be the peptidoglycan, and some form of interaction between this layer and the fibrils might provide the necessary scaffolding for the fibrillar array. In crushed, negatively stained samples of fresh cells, individual fibrils were seen outside the filament, released from the cell wall. These released fibrils were of the same width as those observed in situ but were in short lengths, mostly of 100 to 200 nm, and were invariably bent, sometimes even into U shapes, implying great flexibility. Negative staining of released fibrils showed no evidence that they were hollow tubes but did give some indication of a substructure, implying that they were composed of many subunits. The function of this fibrillar array is unknown, although its position in the cell wall, as well as the correspondence between the angle of the fibrils with respect to the long axis of the filament and the rotation of the filament during gliding, may imply an involvement in gliding motility.
Figures



References
-
- Adams D G. Multicellularity in cyanobacteria. In: Mohan S, Dow C, Cole J A, editors. Prokaryotic structure and function: a new perspective. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 341–384.
-
- Adams D G. Cyanobacteria. In: Shapiro J A, Dworkin M, editors. Bacteria as multicellular organisms. Oxford, United Kingdom: Oxford University Press; 1997. pp. 109–148.
-
- Guglielmi G, Cohen-Bazire G. Structure et distribution des pores et des perforations de l’enveloppe de peptidoglycane chez quelques cyanobactéries. Protistologica. 1982;18:151–165.
-
- Häder D-P, Hoiczyk E. Gliding motility. In: Melkonian M, editor. Algal cell motility. New York, N.Y: Chapman and Hall; 1992. pp. 1–38.
-
- Halfen L N. Gliding motility in Oscillatoria: ultrastructural and chemical characterization of the fibrillar layer. J Phycol. 1973;9:248–253.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

