Effect of wzx (rfbX) mutations on A-band and B-band lipopolysaccharide biosynthesis in Pseudomonas aeruginosa O5
- PMID: 9922263
- PMCID: PMC93466
- DOI: 10.1128/JB.181.3.973-980.1999
Effect of wzx (rfbX) mutations on A-band and B-band lipopolysaccharide biosynthesis in Pseudomonas aeruginosa O5
Abstract
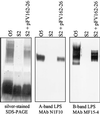
The wbp cluster of Pseudomonas aeruginosa O5 encodes a number of proteins involved in biosynthesis of the heteropolymeric and Wzy-dependent B-band O antigen, including Wzy, the O-antigen polymerase, and Wzz, the regulator of O-antigen chain length. A gene (formerly wbpF), contiguous with wzy in the wbp cluster, is predicted to encode a highly hydrophobic protein with multiple membrane-spanning domains. This secondary structure is consistent with that of Wzx (RfbX), the putative O-antigen unit translocase or "flippase." Insertion of a Gmr cassette at two separate sites within the putative wzx gene led in both cases to the loss of B-band lipopolysaccharide (LPS) O-antigen production. To our knowledge, this is the first report of the successful generation of chromosomal wzx gene replacement mutations. Surprisingly, inactivation of wzx also led to a marked delay in production of the ATP-binding cassette-transporter-dependent, D-rhamnose homopolymer, A-band LPS. This effect on A-band LPS synthesis was alleviated by supplying multiple copies of WbpL in trans. WbpL, a WecA (Rfe) homologue, was shown recently to be essential for the initiation of both A-band and B-band LPS synthesis in P. aeruginosa O5 (H. L. Rocchetta, L. L. Burrows, J. C. Pacan, and J. S. Lam, Mol. Microbiol. 28:1103-1119, 1998). These results suggest that the delay in A-band LPS production may arise from insufficient access to WbpL when the completed B-band O unit is not successfully translocated to the periplasm. Without adequate WbpL, A-band LPS synthesis is delayed. A subset of wzx mutants appeared to have accumulated second-site mutations which either restored the normal expression of A-band LPS or abolished A-band expression completely. Complementation studies showed that all of the additional mutations affecting LPS synthesis that were characterized in this study were located within the B-band LPS genes.
Figures






References
-
- Amor, P. A. Personal communication.
-
- Amor P A, Whitfield C. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 1997;26:145–161. - PubMed
-
- Bastin D A, Stevenson G, Brown P K, Haase A, Reeves P R. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993;7:725–734. - PubMed
-
- Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulphate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

