Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin
- PMID: 9922451
- PMCID: PMC2132888
- DOI: 10.1083/jcb.144.2.241
Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin
Abstract
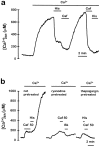
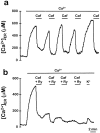
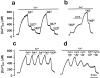
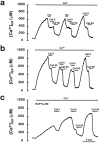
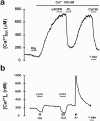
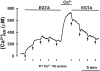
The presence and physiological role of Ca2+-induced Ca2+ release (CICR) in nonmuscle excitable cells has been investigated only indirectly through measurements of cytosolic [Ca2+] ([Ca2+]c). Using targeted aequorin, we have directly monitored [Ca2+] changes inside the ER ([Ca2+]ER) in bovine adrenal chromaffin cells. Ca2+ entry induced by cell depolarization triggered a transient Ca2+ release from the ER that was highly dependent on [Ca2+]ER and sensitized by low concentrations of caffeine. Caffeine-induced Ca2+ release was quantal in nature due to modulation by [Ca2+]ER. Whereas caffeine released essentially all the Ca2+ from the ER, inositol 1,4, 5-trisphosphate (InsP3)- producing agonists released only 60-80%. Both InsP3 and caffeine emptied completely the ER in digitonin-permeabilized cells whereas cyclic ADP-ribose had no effect. Ryanodine induced permanent emptying of the Ca2+ stores in a use-dependent manner after activation by caffeine. Fast confocal [Ca2+]c measurements showed that the wave of [Ca2+]c induced by 100-ms depolarizing pulses in voltage-clamped cells was delayed and reduced in intensity in ryanodine-treated cells. Our results indicate that the ER of chromaffin cells behaves mostly as a single homogeneous thapsigargin-sensitive Ca2+ pool that can release Ca2+ both via InsP3 receptors or CICR.
Figures



References
-
- Albillos A, Garcia AG, Olivera B, Gandia L. Re-evaluation of the P/Q Ca2+ channel components of Ba2+ currents in bovine chromaffin cells superfused with solutions containing low and high Ba2+concentrations. Pflug Arch Eur J Physiol. 1996;432:1030–1038. - PubMed
-
- Alonso MT, Barrero MJ, Carnicero E, Montero M, Garcia-Sancho J, Alvarez J. Functional measurements of [Ca2+] in the endoplasmic reticulum using a herpes virus to deliver targeted aequorin. Cell Calcium. 1998;24:87–96. - PubMed
-
- Barrero MJ, Montero M, Alvarez J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. A comparative study. J Biol Chem. 1997;272:27694–27699. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Berridge MJ. Neuronal calcium signalling. Neuron. 1998;21:13–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

