Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets
- PMID: 9922458
- PMCID: PMC2132899
- DOI: 10.1083/jcb.144.2.325
Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets
Abstract
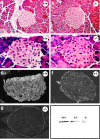
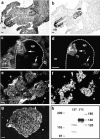
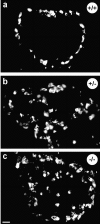
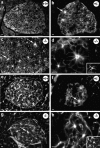
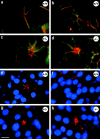
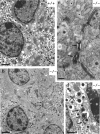
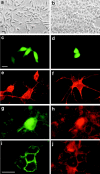
Classical cell dissociation/reaggregation experiments with embryonic tissue and cultured cells have established that cellular cohesiveness, mediated by cell adhesion molecules, is important in determining the organization of cells within tissue and organs. We have employed N-CAM-deficient mice to determine whether N-CAM plays a functional role in the proper segregation of cells during the development of islets of Langerhans. In N-CAM-deficient mice the normal localization of glucagon-producing alpha cells in the periphery of pancreatic islets is lost, resulting in a more randomized cell distribution. In contrast to the expected reduction of cell-cell adhesion in N-CAM-deficient mice, a significant increase in the clustering of cadherins, F-actin, and cell-cell junctions is observed suggesting enhanced cadherin-mediated adhesion in the absence of proper N-CAM function. These data together with the polarized distribution of islet cell nuclei and Na+/K+-ATPase indicate that islet cell polarity is also affected. Finally, degranulation of beta cells suggests that N-CAM is required for normal turnover of insulin-containing secretory granules. Taken together, our results confirm in vivo the hypothesis that a cell adhesion molecule, in this case N-CAM, is required for cell type segregation during organogenesis. Possible mechanisms underlying this phenomenon may include changes in cadherin-mediated adhesion and cell polarity.
Figures
References
-
- Bosco D, Orci L, Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res. 1989;184:72–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

