Reversible Ca gradients between the subplasmalemma and cytosol differentially activate Ca-dependent Cl currents
- PMID: 9925823
- PMCID: PMC2223373
- DOI: 10.1085/jgp.113.2.249
Reversible Ca gradients between the subplasmalemma and cytosol differentially activate Ca-dependent Cl currents
Abstract
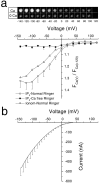
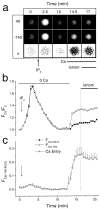
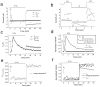
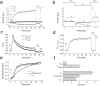
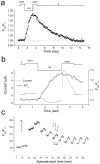
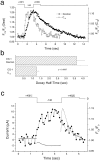
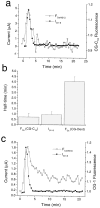
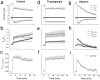
Xenopus oocytes express several different Ca-activated Cl currents that have different waveforms and biophysical properties. We compared the stimulation of Ca-activated Cl currents measured by two-microelectrode voltage clamp with the Ca transients measured in the same cell by confocal microscopy and Ca-sensitive fluorophores. The purpose was to determine how the amplitude and/or spatio-temporal features of the Ca signal might explain how these different Cl currents were activated by Ca. Because Ca release from stores was voltage independent, whereas Ca influx depended upon the electrochemical driving force, we were able to separately assess the contribution of Ca from these two sources. We were surprised to find that Ca signals measured with a cytosolic Ca-sensitive dye, dextran-conjugated Ca-green-1, correlated poorly with Cl currents. This suggested that Cl channels located at the plasma membrane and the Ca-sensitive dye located in the bulk cytosol were sensing different [Ca]. This was true despite Ca measurement in a confocal slice very close to the plasma membrane. In contrast, a membrane-targeted Ca-sensitive dye (Ca-green-C18) reported a Ca signal that correlated much more closely with the Cl currents. We hypothesize that very local, transient, reversible Ca gradients develop between the subplasmalemmal space and the bulk cytosol. [Ca] is higher near the plasma membrane when Ca is provided by Ca influx, whereas the gradient is reversed when Ca is released from stores, because Ca efflux across the plasma membrane is faster than diffusion of Ca from the bulk cytosol to the subplasmalemmal space. Because dissipation of the gradients is accelerated by inhibition of Ca sequestration into the endoplasmic reticulum with thapsigargin, we conclude that [Ca] in the bulk cytosol declines slowly partly due to futile recycling of Ca through the endoplasmic reticulum.
Figures



Similar articles
-
Activation of different Cl currents in Xenopus oocytes by Ca liberated from stores and by capacitative Ca influx.J Gen Physiol. 1996 Sep;108(3):157-75. doi: 10.1085/jgp.108.3.157. J Gen Physiol. 1996. PMID: 8882861 Free PMC article.
-
Ca(2+)-dependent activation of Cl(-) currents in Xenopus oocytes is modulated by voltage.Am J Physiol Cell Physiol. 2000 Apr;278(4):C667-75. doi: 10.1152/ajpcell.2000.278.4.C667. Am J Physiol Cell Physiol. 2000. PMID: 10751316
-
Asymmetrical distribution of Ca-activated Cl channels in Xenopus oocytes.Biophys J. 1998 Mar;74(3):1286-95. doi: 10.1016/S0006-3495(98)77842-7. Biophys J. 1998. PMID: 9512026 Free PMC article.
-
Imaging calcium entering the cytosol through a single opening of plasma membrane ion channels: SCCaFTs--fundamental calcium events.Cell Calcium. 2004 Jun;35(6):523-33. doi: 10.1016/j.ceca.2004.01.019. Cell Calcium. 2004. PMID: 15110142 Review.
-
Techniques for measuring and manipulating free Ca2+ in the cytosol and organelles of neutrophils.J Immunol Methods. 1999 Dec 17;232(1-2):77-88. doi: 10.1016/s0022-1759(99)00167-2. J Immunol Methods. 1999. PMID: 10618511 Review.
Cited by
-
Ca2+-calmodulin-dependent protein kinase II potentiates store-operated Ca2+ current.J Biol Chem. 2003 Sep 5;278(36):33730-7. doi: 10.1074/jbc.M305023200. Epub 2003 Jun 22. J Biol Chem. 2003. PMID: 12821654 Free PMC article.
-
Modulation of Intracellular ROS and Senescence-Associated Phenotypes of Xenopus Oocytes and Eggs by Selective Antioxidants.Antioxidants (Basel). 2021 Jul 1;10(7):1068. doi: 10.3390/antiox10071068. Antioxidants (Basel). 2021. PMID: 34356301 Free PMC article.
-
The carboxy terminal coiled-coil modulates Orai1 internalization during meiosis.Sci Rep. 2021 Jan 27;11(1):2290. doi: 10.1038/s41598-021-82048-z. Sci Rep. 2021. PMID: 33504898 Free PMC article.
-
Calcium dynamics during physiological acidification in Xenopus oocyte.J Membr Biol. 2010 Aug;236(3):233-45. doi: 10.1007/s00232-010-9290-1. Epub 2010 Aug 18. J Membr Biol. 2010. PMID: 20717657
-
GmN70 and LjN70. Anion transporters of the symbiosome membrane of nodules with a transport preference for nitrate.Plant Physiol. 2005 Apr;137(4):1435-44. doi: 10.1104/pp.104.051953. Epub 2005 Mar 25. Plant Physiol. 2005. PMID: 15793072 Free PMC article.
References
-
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. - PubMed
-
- Berg, H.C. 1993. Random Walks in Biology. Princeton University Press, Princeton, NJ. 152 pp.
-
- Berridge MJ. The AM and FM of calcium signalling. Nature. 1997b;386:759–760. - PubMed

