N-linked glycosylation sites determine HERG channel surface membrane expression
- PMID: 9925876
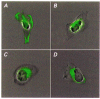
- PMCID: PMC2269130
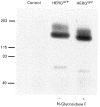
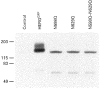
- DOI: 10.1111/j.1469-7793.1999.041ad.x
N-linked glycosylation sites determine HERG channel surface membrane expression
Abstract
1. Long QT syndrome (LQT) is an electrophysiological disorder that can lead to sudden death from cardiac arrhythmias. One form of LQT has been attributed to mutations in the human ether-a-go-go-related gene (HERG) that encodes a voltage-gated cardiac K+ channel. While a recent report indicates that LQT in some patients is associated with a mutation of HERG at a consensus extracellular N-linked glycosylation site (N629), earlier studies failed to identify a role for N-linked glycosylation in the functional expression of voltage-gated K+ channels. In this study we used pharmacological agents and site-directed mutagenesis to assess the contribution of N-linked glycosylation to the surface localization of HERG channels. 2. Tunicamycin, an inhibitor of N-linked glycosylation, blocked normal surface membrane expression of a HERG-green fluorescent protein (GFP) fusion protein (HERGGFP) transiently expressed in human embryonic kidney (HEK 293) cells imaged with confocal microscopy. 3. Immunoblot analysis revealed that N-glycosidase F shifted the molecular mass of HERGGFP, stably expressed in HEK 293 cells, indicating the presence of N-linked carbohydrate moieties. Mutations at each of the two putative extracellular N-linked glycosylation sites (N598Q and N629Q) led to a perinuclear subcellular localization of HERGGFP stably expressed in HEK 293 cells, with no surface membrane expression. Furthermore, patch clamp analysis revealed that there was a virtual absence of HERG current in the N-glycosylation mutants. 4. Taken together, these results strongly suggest that N-linked glycosylation is required for surface membrane expression of HERG. These findings may provide insight into a mechanism responsible for LQT2 due to N-linked glycosylation-related mutations of HERG.
Figures


References
-
- Akimoto K, Furutani M, Kasanuki H, Imammura S-I, Furutani Y, Takao A, Monma K, Matsuoka R. Coexistence of missense mutation of HERG and mitochondrial DNA in Japanese long QT family. Circulation. 1996;94:I164.
-
- Benson DW, MacRae CA, Vesely MR, Walsh EP, Seidman JG, Seidman CE, Satler CA. Missense mutation in the pore region of HERG causes familial long QT syndrome. Circulation. 1996;93:1791–1795. - PubMed
-
- Bonifacino JS, Lippincot-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Current Opinion in Cell Biology. 1991;7:592–600. - PubMed
-
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia. HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. - PubMed
-
- Dausse E, Berthet M, Denjoy I, Andre-Fouet X, Cruaud C, Bennaceur M, Faure S, Coumel P, Schwartz K, Guicheney P. A mutation in HERG associated with notched T waves in long QT syndrome. Journal of Molecular and Cellular Cardiology. 1996;28:1609–1615. 10.1006/jmcc.1996.0151. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

