Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide
- PMID: 9927500
- PMCID: PMC407899
- DOI: 10.1172/JCI4890
Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide
Abstract
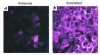
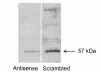
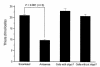
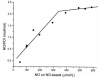
Since thiols can undergo nitrosation and the cell membrane is rich in thiol-containing proteins, we considered the possibility that membrane surface thiols may regulate cellular entry of NO. Recently, protein disulfide isomerase (PDI), a protein that catalyzes thio-disulfide exchange reactions, has been found on the cell-surface membrane. We hypothesized that cell-surface PDI reacts with NO, catalyzes S-nitrosation reactions, and facilitates NO transfer from the extracellular to intracellular compartment. We observed that PDI catalyzes the S-nitrosothiol-dependent oxidation of the heme group of myoglobin (15-fold increase in the rate of oxidation compared with control), and that NO reduces the activity of PDI by 73.1 +/- 21.8% (P < 0.005). To assess the role of PDI in the cellular action of NO, we inhibited human erythroleukemia (HEL) cell-surface PDI expression using an antisense phosphorothioate oligodeoxynucleotide directed against PDI mRNA. This oligodeoxynucleotide decreased cell-surface PDI content by 74.1 +/- 9.3% and PDI folding activity by 46.6 +/- 3.5% compared with untreated or "scrambled" phosphorothioate oligodeoxynucleotide-treated cells (P < 0.0001). This decrease in cell-surface PDI was associated with a significant decrease in cyclic guanosine monophosphate (cGMP) generation after S-nitrosothiol exposure (65.4 +/- 26.7% reduction compared with control; P < 0.05), with no effect on cyclic adenosine monophosphate (cAMP) generation after prostaglandin E1 exposure. These data demonstrate that the cellular entry of NO involves a transnitrosation mechanism catalyzed by cell-surface PDI. These observations suggest a unique mechanism by which extracellular NO gains access to the intracellular environment.
Figures




References
-
- Koch G. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1985;87:491–492. - PubMed
-
- Creighton TE, Hillson D, Freedman R. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol. 1980;142:43–62. - PubMed
-
- Gilbert H. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. - PubMed
-
- Gilbert H. Protein disulfide isomerase and assisted protein folding. J Biol Chem. 1997;272:29399–29402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

