Immune responses to Ro60 and its peptides in mice. I. The nature of the immunogen and endogenous autoantigen determine the specificities of the induced autoantibodies
- PMID: 9927515
- PMCID: PMC2192918
- DOI: 10.1084/jem.189.3.531
Immune responses to Ro60 and its peptides in mice. I. The nature of the immunogen and endogenous autoantigen determine the specificities of the induced autoantibodies
Abstract
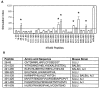
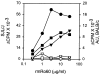
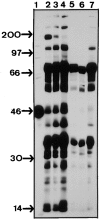
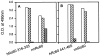
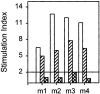
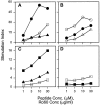
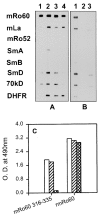
Anti-Ro60 autoantibodies are found in a variety of autoimmune disorders including systemic lupus erythematosus (SLE), Sjögren's syndrome, primary biliary cirrhosis, and active hepatitis. They are the most prevalent autoantibodies in normal individuals and in asymptomatic mothers of infants afflicted with neonatal lupus. In the present study, immune responses to recombinant human Ro60 (rhRo60) and recombinant mouse Ro60 (rmRo60) and selected Ro60 peptides in non-SLE-prone mice were investigated. Multiple T and B cell epitopes were identified in Ro60. Immunizations with either xenogeneic or autologous Ro60 induced autoantibodies to a diverse group of autoantigens. In addition to La and Ro52, proteins in the small nuclear ribonucleoprotein (snRNP) particles such as SmA, SmB, SmD, and 70-kD U1-RNP were unexpectedly identified as targeted antigens. In the studies involving synthetic Ro60 peptides, both human and mouse Ro60316-335 peptides, which differ in three amino acids, were found to contain dominant cross-reactive T cell determinants. Immunizations with these peptides induced autoantibodies to Ro60, La, SmD, and 70-kD U1-RNP without autoantibodies to Ro52, SmA, or SmB. With human Ro60316-335 as the immunogen, additional autoantibodies reactive with the Golgi complex were found. In contrast to the immunodominance of both human and mouse Ro60316-335 peptides, the T cell determinant in human Ro60441-465 was dominant, whereas that in the mouse peptide was cryptic. Immunization with human Ro60441-465 induced primarily anti-peptide Abs. Mouse Ro60441-465 failed to induce an antibody response. These results show that both the nature of the immunogen and the immunogenicity of the related endogenous antigen are important in determining the specificities of the autoantibodies generated. They have significant implications for proposed mechanisms on the generation of complex patterns of autoantibodies to a diverse group of autoantigens in SLE patients.
Figures







References
-
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus (SLE) Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune disease and probes for cell biopsy. Adv Immunol. 1989;44:93–151. - PubMed
-
- Reichlin, M., and J.B. Harley. 1997. Antinuclear antibodies: an overview. In Dubois' Lupus Erythematosus. D.J. Wallace and B.H. Hahn, editors. Williams and Wilkins, Baltimore, MD. 397–405.
-
- Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren's syndrome and systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:337–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

