Xenopus laevis sperm receptor gp69/64 glycoprotein is a homolog of the mammalian sperm receptor ZP2
- PMID: 9927653
- PMCID: PMC15310
- DOI: 10.1073/pnas.96.3.829
Xenopus laevis sperm receptor gp69/64 glycoprotein is a homolog of the mammalian sperm receptor ZP2
Abstract
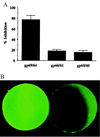
Little is known about sperm-binding proteins in the egg envelope of nonmammalian vertebrate species. We report here the molecular cloning and characterization of a recently identified sperm receptor (gp69/64) in the Xenopus laevis egg vitelline envelope. Our data indicate that the gp69 and gp64 glycoproteins are two glycoforms of the receptor and have the same number of N-linked oligosaccharide chains but differ in the extent of O-glycosylation. The amino acid sequence of the receptor is closely related to that of the mouse zona pellucida protein ZP2. Most of the sequence conservation, including a ZP domain, a potential furin cleavage site, and a putative transmembrane domain are located in the C-terminal half of the receptor. Proteolytic cleavage of the gp69/64 protein by a cortical granule protease during fertilization removes 27 amino acid residues from the N terminus of gp69/64 and results in loss of sperm binding to the activated eggs. Similarly, we find that treatment of eggs with type I collagenase removes 31 residues from the N terminus of gp69/64 and has the same effect on sperm binding. The isolated and purified N terminus-truncated receptor protein is inactive as an inhibitor of sperm-egg binding. Earlier studies on the effect of Pronase digestion on receptor activity suggest that this N-terminal peptide may contain an O-linked glycan that is involved in the binding process. Based on these results and the findings on the primary structure of the receptor, a pathway for the maturation and secretion of gp69/64, as well as its inactivation following fertilization, is proposed.
Figures
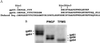


References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

