Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes
- PMID: 9927675
- PMCID: PMC15332
- DOI: 10.1073/pnas.96.3.956
Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes
Abstract
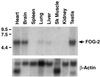
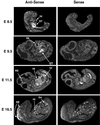
GATA transcription factors are important regulators of both hematopoiesis (GATA-1/2/3) and cardiogenesis (GATA-4) in mammals. The transcriptional activities of the GATA proteins are modulated by their interactions with other transcription factors and with transcriptional coactivators and repressors. Recently, two related zinc finger proteins, U-shaped (USH) and Friend of GATA-1 (FOG) have been reported to interact with the GATA proteins Pannier and GATA-1, respectively, and to modulate their transcriptional activities in vitro and in vivo. In this report, we describe the molecular cloning and characterization of a third FOG-related protein, FOG-2. FOG-2 is an 1,151 amino acid nuclear protein that contains eight zinc finger motifs that are structurally related to those of both FOG and USH. FOG-2 is first expressed in the mouse embryonic heart and septum transversum at embryonic day 8.5 and is subsequently expressed in the developing neuroepithelium and urogenital ridge. In the adult, FOG-2 is expressed predominately in the heart, brain, and testis. FOG-2 associates physically with the N-terminal zinc finger of GATA-4 both in vitro and in vivo. This interaction appears to modulate specifically the transcriptional activity of GATA-4 because overexpression of FOG-2 in both NIH 3T3 cells and primary rat cardiomyocytes represses GATA-4-dependent transcription from multiple cardiac-restricted promoters. Taken together, these results implicate FOG-2 as a novel modulator of GATA-4 function during cardiac development and suggest a paradigm in which tissue-specific interactions between different FOG and GATA proteins regulate the differentiation of distinct mesodermal cell lineages.
Figures

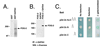

References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

