T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation
- PMID: 9927687
- PMCID: PMC15344
- DOI: 10.1073/pnas.96.3.1024
T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation
Abstract
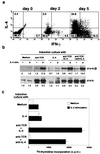
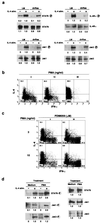
The central role of type-2 helper T (Th2) cells in the development of allergic responses and immune responses against helminthic parasites is well documented. The differentiation of Th2 cells from naive T cells requires both the recognition of antigen by T cell antigen receptors (TCR) and the activation of downstream signal-transduction molecules of the interleukin 4 receptor (IL-4R) pathway, including Jak1, Jak3, and STAT6. Little is known, however, about how these two distinct pathways cooperate with each other to induce Th2 cells. Here, we use a T cell-specific H-Ras-dominant-negative transgenic mouse to show that TCR-mediated activation of the Ras/mitogen-activated protein kinase pathway alters IL-4R function and is required for Th2 cell differentiation. The enhancement of IL-4R signaling seems to be a consequence of both direct "crosstalk" with the TCR signaling pathway and increased protein expression of downstream signaling molecules of the IL-4R pathway. Therefore, successful Th2 differentiation depends on the effectiveness of the TCR-mediated activation of the Ras/mitogen-activated protein kinase pathway in modifying the IL-4R-mediated signaling pathway.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

