Linomide suppresses experimental autoimmune neuritis in Lewis rats by inhibiting myelin antigen-reactive T and B cell responses
- PMID: 9933420
- PMCID: PMC1905200
- DOI: 10.1046/j.1365-2249.1999.00768.x
Linomide suppresses experimental autoimmune neuritis in Lewis rats by inhibiting myelin antigen-reactive T and B cell responses
Abstract
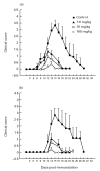
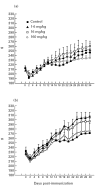
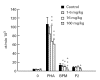
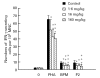
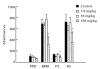
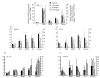
Linomide (quinoline-3-carboxamide) is a synthetic immunomodulator that suppresses several experimental autoimmune diseases. Here we report the effects of Linomide on experimental autoimmune neuritis (EAN), a CD4+ T cell-mediated animal model of acute Guillain-Barré syndrome (GBS) in humans. EAN induced in Lewis rats by inoculation with bovine peripheral nervous system (PNS) myelin and Freund's complete adjuvant was strongly suppressed by Linomide administered daily subcutaneously from the day of inoculation. Linomide dose-dependently delayed the interval between immunization and onset of clinical EAN, as well as the severity of EAN symptoms. These clinical effects were associated with dose-dependent down-modulation of PNS antigen-induced T and B cell responses and with suppression of the proinflammatory cytokines IL-12, interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha) mRNA. In PNS sections, Linomide suppressed IL-12 and TNF-alpha, and up-regulated IL-10 mRNA expression. These findings suggest that Linomide could be useful in certain T cell-dependent autoimmune diseases.
Figures
Similar articles
-
Resistance and susceptibility to experimental autoimmune neuritis in Sprague-Dawley and Lewis rats correlate with different levels of autoreactive T and B cell responses to myelin antigens.J Neurosci Res. 1998 Nov 1;54(3):373-81. doi: 10.1002/(SICI)1097-4547(19981101)54:3<373::AID-JNR8>3.0.CO;2-Z. J Neurosci Res. 1998. PMID: 9819142
-
Linomide-induced suppression of experimental autoimmune neuritis is associated with down-regulated macrophage functions.J Neuroimmunol. 1997 Jun;76(1-2):177-84. doi: 10.1016/s0165-5728(97)00051-9. J Neuroimmunol. 1997. PMID: 9184648
-
Suppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissue.Neuropharmacology. 2002 Apr;42(5):731-9. doi: 10.1016/s0028-3908(02)00015-1. Neuropharmacology. 2002. PMID: 11985832
-
The effects of monoamine reuptake inhibiting antidepressants in experimental allergic neuritis.J Peripher Nerv Syst. 1997;2(1):30-42. J Peripher Nerv Syst. 1997. PMID: 10975734 Review.
-
Effects of Linomide on immune cells and cytokines inhibit autoimmune pathologies of the central and peripheral nervous system.Int Immunopharmacol. 2001 Jun;1(6):1123-30. doi: 10.1016/s1567-5769(01)00041-8. Int Immunopharmacol. 2001. PMID: 11407306 Review.
Cited by
-
The strategies used for treatment of experimental autoimmune neuritis (EAN): a beneficial effect of glatiramer acetate administered intraperitoneally.Clin Rev Allergy Immunol. 2012 Apr;42(2):181-8. doi: 10.1007/s12016-010-8246-7. Clin Rev Allergy Immunol. 2012. PMID: 21234710
-
Biological Drugs in Guillain-Barré Syndrome: An Update.Curr Neuropharmacol. 2017;15(7):938-950. doi: 10.2174/1570159X14666161213114904. Curr Neuropharmacol. 2017. PMID: 27964705 Free PMC article. Review.
-
Attenuated EAN in TNF-α deficient mice is associated with an altered balance of M1/M2 macrophages.PLoS One. 2012;7(5):e38157. doi: 10.1371/journal.pone.0038157. Epub 2012 May 30. PLoS One. 2012. PMID: 22666471 Free PMC article.
-
The critical role of IL-12p40 in initiating, enhancing, and perpetuating pathogenic events in murine experimental autoimmune neuritis.Brain Pathol. 2002 Oct;12(4):420-9. doi: 10.1111/j.1750-3639.2002.tb00459.x. Brain Pathol. 2002. PMID: 12408228 Free PMC article.
References
-
- Hartung HP, Pollard JD, Harvey GK, Toyka KV. Immunopathogenesis and treatment of the Guillain–Barré syndrome. Part I. Muscle and Nerve. 1995;18:137–53.. - PubMed
-
- McCombe PA. Autoimmune diseases. In: Pender MP, McCombe PA, editors. Autoimmune neurological diseases. Cambridge: Cambridge University Press; 1995. pp. 177–228.
-
- Linington C, Izumo S, Suzuki M, Uyemura K, Meyermann R, Wekerle H. A permanent rat T cell line that mediates experimental allergic neuritis in the Lewis rat in vivo. J Immunol. 1984;133:1946–50. - PubMed
-
- Linington C, Lassmann H, Ozawa K, Kosin S, Mongan L. Cell adhesion molecules of the immunoglobulin supergene family as tissue-specific autoantigens: induction of experimental allergic neuritis (EAN) by P0 protein-specific T cell lines. Eur J Immunol. 1992;22:1813–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

