Administration of intravenous immunoglobulin (IVIG) in vivo--down-regulatory effects on the IL-1 system
- PMID: 9933433
- PMCID: PMC1905185
- DOI: 10.1046/j.1365-2249.1999.00757.x
Administration of intravenous immunoglobulin (IVIG) in vivo--down-regulatory effects on the IL-1 system
Abstract
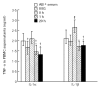
Modulation of the cytokine network may be of importance for the beneficial effects of therapy with IVIG seen in a wide range of immune-mediated disorders. In the present study we investigate the effect of IVIG administration in vivo on the IL-1 system in 12 patients with primary hypogammaglobulinaemia. Before IVIG infusion these patients had significantly elevated levels of IL-1alpha and IL-1beta both in plasma and in supernatants from peripheral blood mononuclear cells (PBMC) compared with healthy controls. After one bolus infusion with IVIG (0.4 g/kg) we found a significant change in the profile of the components of the IL-1 system: a marked increase in levels of IL-1 receptor antagonist (IL-1Ra) and neutralizing antibodies against IL-1alpha, a moderate decrease in levels of IL-1alpha, IL-1beta and soluble (s) IL-1 receptor type I and a significant increase in sIL-1 receptor type II levels. These changes were found both in plasma and in PBMC isolated after IVIG administration. Furthermore, pooled serum obtained after IVIG infusion suppressed lipopolysaccharide- and staphylococcal enterotoxin B-stimulated, but not phorbol myristate acetate-stimulated, release of IL-1alpha and IL-1beta from PBMC isolated from healthy controls. Finally, these changes in circulating levels of various IL-1 modulators after IVIG infusion appeared to cause a significantly impaired ability of IL-1 to stimulate PBMC for tumour necrosis factor-alpha release. Our findings suggest that IVIG administration may not only down-regulate the activity in the IL-1 system, but also hamper IL-1 stimulation of PBMC.
Figures

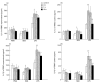
Similar articles
-
Interleukin 1 components in cicatricial pemphigoid. Role in intravenous immunoglobulin therapy.Cytokine. 2001 May 21;14(4):218-24. doi: 10.1006/cyto.2001.0877. Cytokine. 2001. PMID: 11448121
-
Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo.Blood. 1994 Oct 1;84(7):2136-43. Blood. 1994. PMID: 7919327
-
Pemphigus vulgaris: the role of IL-1 and IL-1 receptor antagonist in pathogenesis and effects of intravenous immunoglobulin on their production.Clin Immunol. 2001 Aug;100(2):172-80. doi: 10.1006/clim.2001.5061. Clin Immunol. 2001. PMID: 11465946
-
Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages.Clin Exp Immunol. 1996 May;104 Suppl 1:10-20. Clin Exp Immunol. 1996. PMID: 8625537
-
Pretreatment cytokine profiles of peripheral blood mononuclear cells and serum from patients with rheumatoid arthritis in different american college of rheumatology response groups to methotrexate.J Rheumatol. 2003 Jan;30(1):28-35. J Rheumatol. 2003. PMID: 12508386
Cited by
-
Intravenous Immunoglobulin for Overwhelming Postsplenectomy Infection.J Glob Infect Dis. 2021 Jan 29;13(1):44-51. doi: 10.4103/jgid.jgid_93_19. eCollection 2021 Jan-Mar. J Glob Infect Dis. 2021. PMID: 33911454 Free PMC article.
-
Mechanistic effects of IVIg in neuroinflammatory diseases: conclusions based on clinicopathologic correlations.J Clin Immunol. 2014 Jul;34 Suppl 1:S120-6. doi: 10.1007/s10875-014-0024-5. Epub 2014 Apr 11. J Clin Immunol. 2014. PMID: 24722854 Review.
-
The efficiency of intraperitoneal high-dose immunoglobulin in experimental nephrotic syndrome.Pediatr Nephrol. 2006 Jan;21(1):39-45. doi: 10.1007/s00467-005-2046-y. Epub 2005 Oct 27. Pediatr Nephrol. 2006. PMID: 16252108
-
Neuroinflammatory Nexus of Pediatric Epilepsy.J Pediatr Epilepsy. 2018 Jun;7(2):32-39. doi: 10.1055/s-0038-1668601. Epub 2018 Sep 3. J Pediatr Epilepsy. 2018. PMID: 31709125 Free PMC article.
-
Bench-to-bedside review: Immunoglobulin therapy for sepsis - biological plausibility from a critical care perspective.Crit Care. 2012 Dec 12;16(2):206. doi: 10.1186/cc10597. Crit Care. 2012. PMID: 22424150 Free PMC article. Review.
References
-
- Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med. 1992;326:107–16. - PubMed
-
- Mobini N, Sarela A, Ahmed AR. Intravenous immunoglobulins in the therapy of autoimmune and systemic inflammatory disorders. Ann Allergy Asthma Immunol. 1995;74:119–28. - PubMed
-
- Dalakas MC. Intravenous immune globulin therapy for neurologic disease. Ann Intern Med. 1997;126:721–30. - PubMed
-
- Wolf HM, Eibl MM. Immunomodulatory effect of immunoglobulins. Clin Exp Rheumatol. 1996;14(Suppl. 15):S17–S25. - PubMed
-
- Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol. 1997;100:151–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

