Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts
- PMID: 9933439
- PMCID: PMC1905193
- DOI: 10.1046/j.1365-2249.1999.00775.x
Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts
Abstract
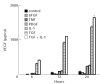
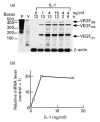
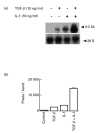
Vascular endothelial growth factor (VEGF) is abundant in synovium and synovial fluids, where it probably contributes to vascular permeability and angiogenesis in arthritic joints. To investigate the probable sources of VEGF in synovium, we compared the ability of several cytokines (TGF-beta, platelet-derived growth factor (PDGF), IL-1, tumour necrosis factor (TNF), basic fibroblast growth factor (bFGF) that are associated with arthritis and angiogenesis, to stimulate secretion of VEGF protein by human synovial fibroblasts. TGF-beta was the strongest inducer of VEGF secretion; six times more VEGF was secreted when cells were stimulated by TGF-beta than when stimulated by PDGF or IL-1 for 24 h. TNF-alpha and bFGF did not stimulate any secretion of VEGF. The stimulatory effects of TGF-beta and IL-1 on VEGF secretion were additive. Hypoxic culture alone also stimulated VEGF secretion, but more importantly, hypoxic culture conditions doubled the rate of VEGF secretion stimulated by the cytokines TGF-beta and IL-1. When dermal and synovial fibroblasts were stimulated identically by hypoxia and cytokines (TGF-beta and IL-1), synovial fibroblasts secreted four times more VEGF than did dermal fibroblasts. Thus in rheumatoid arthritis, the capacity of synovial fibroblasts in the hypoxic environment to secrete large amounts of VEGF in response to cytokines such as TGF-beta probably contributes significantly to angiogenesis in the synovium.
Figures

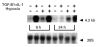

Similar articles
-
Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts.Arthritis Rheum. 2002 Oct;46(10):2587-97. doi: 10.1002/art.10520. Arthritis Rheum. 2002. PMID: 12384916
-
Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta.J Rheumatol. 1997 Jul;24(7):1253-9. J Rheumatol. 1997. PMID: 9228120
-
CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor.J Immunol. 2000 May 15;164(10):5055-61. doi: 10.4049/jimmunol.164.10.5055. J Immunol. 2000. PMID: 10799861
-
Synergistic upregulation of ADAMTS4 (aggrecanase-1) by cytokines and its suppression in knee osteoarthritic synovial fibroblasts.Lab Invest. 2022 Jan;102(1):102-111. doi: 10.1038/s41374-021-00685-4. Epub 2021 Oct 30. Lab Invest. 2022. PMID: 34718343
-
Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only.Circulation. 1994 Aug;90(2):649-52. doi: 10.1161/01.cir.90.2.649. Circulation. 1994. PMID: 8044933
Cited by
-
Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do!Ann Rheum Dis. 2005 Jul;64(7):971-80. doi: 10.1136/ard.2004.031641. Epub 2005 Mar 30. Ann Rheum Dis. 2005. PMID: 15800008 Free PMC article. Review.
-
VEGF and imaging of vessels in rheumatoid arthritis.Arthritis Res. 2002;4 Suppl 3(Suppl 3):S99-107. doi: 10.1186/ar582. Epub 2002 May 9. Arthritis Res. 2002. PMID: 12110128 Free PMC article. Review.
-
Organ Fibrosis and Autoimmunity: The Role of Inflammation in TGFβ-Dependent EMT.Biomolecules. 2021 Feb 18;11(2):310. doi: 10.3390/biom11020310. Biomolecules. 2021. PMID: 33670735 Free PMC article. Review.
-
Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts.Clin Exp Immunol. 2007 Mar;147(3):573-9. doi: 10.1111/j.1365-2249.2006.03295.x. Clin Exp Immunol. 2007. PMID: 17302909 Free PMC article.
-
Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment.Matrix Biol. 2016 Apr;51:37-46. doi: 10.1016/j.matbio.2016.01.013. Epub 2016 Feb 2. Matrix Biol. 2016. PMID: 26844756 Free PMC article. Review.
References
-
- Koch A. Angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41:951–62. - PubMed
-
- Paleolog E, Fava RA. Angiogenesis in rheumatoid arthritis. Spring Sem Immunol. 1998 in press. - PubMed
-
- Ferrara N, Davis-Srnyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

