Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors
- PMID: 9952395
- PMCID: PMC6786025
- DOI: 10.1523/JNEUROSCI.19-04-01165.1999
Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors
Abstract
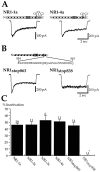
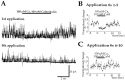
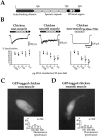
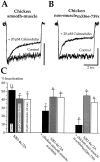
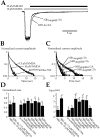
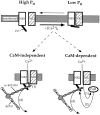
Glutamate receptors are associated with various regulatory and cytoskeletal proteins. However, an understanding of the functional significance of these interactions is still rudimentary. Studies in hippocampal neurons suggest that such interactions may be involved in calcium-induced reduction in the open probability of NMDA receptors (inactivation). Thus we examined the role of the intracellular domains of the NR1 subunit and two of its binding partners, calmodulin and alpha-actinin, on this process using NR1/NR2A heteromers expressed in human embryonic kidney (HEK) 293 cells. The presence of the first 30 residues of the intracellular C terminus of NR1 (C0 domain) was required for inactivation. Mutations in the last five residues of C0 reduced inactivation and produced parallel shifts in binding of alpha-actinin and Ca2+/calmodulin to the respective C0-derived peptides. Although calmodulin reduced channel activity in excised patches, calmodulin inhibitors did not block inactivation in whole-cell recording, suggesting that inactivation in the intact cell is more complex than binding of calmodulin to C0. Overexpression of putative Ca2+-insensitive, but not Ca2+-sensitive, forms of alpha-actinin reduced inactivation, an effect that was overcome by inclusion of calmodulin in the whole-cell pipette. The C0 domain also directly affects channel gating because NR1 subunits with truncated C0 domains that lacked calmodulin or alpha-actinin binding sites had a low open probability. We propose that inactivation can occur after C0 dissociates from alpha-actinin by two distinct but converging calcium-dependent processes: competitive displacement of alpha-actinin by calmodulin and reduction in the affinity of alpha-actinin for C0 after binding of calcium to alpha-actinin.
Figures




Similar articles
-
Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding.Biochemistry. 2007 Jul 24;46(29):8485-97. doi: 10.1021/bi0623025. Epub 2007 Jun 30. Biochemistry. 2007. PMID: 17602661 Free PMC article.
-
Reduced ethanol inhibition of N-methyl-D-aspartate receptors by deletion of the NR1 C0 domain or overexpression of alpha-actinin-2 proteins.J Biol Chem. 2000 May 19;275(20):15019-24. doi: 10.1074/jbc.275.20.15019. J Biol Chem. 2000. PMID: 10809744
-
Regulation of single NMDA receptor channel activity by alpha-actinin and calmodulin in rat hippocampal granule cells.J Physiol. 2004 Jun 15;557(Pt 3):795-808. doi: 10.1113/jphysiol.2003.059212. Epub 2004 Apr 8. J Physiol. 2004. PMID: 15073274 Free PMC article.
-
Inhibitory interactions of calcineurin (phosphatase 2B) and calmodulin on rat hippocampal NMDA receptors.Neuropharmacology. 2004 Sep;47(4):505-14. doi: 10.1016/j.neuropharm.2004.06.001. Neuropharmacology. 2004. PMID: 15380369
-
Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors.Neuron. 1998 Aug;21(2):443-53. doi: 10.1016/s0896-6273(00)80553-x. Neuron. 1998. PMID: 9728925
Cited by
-
Chemical shift assignments of the α-actinin C-terminal EF-hand domain bound to a cytosolic C0 domain of GluN1 (residues 841-865) from the NMDA receptor.Biomol NMR Assign. 2024 Dec;18(2):239-244. doi: 10.1007/s12104-024-10194-2. Epub 2024 Aug 29. Biomol NMR Assign. 2024. PMID: 39207574 Free PMC article.
-
The DREAM protein negatively regulates the NMDA receptor through interaction with the NR1 subunit.J Neurosci. 2010 Jun 2;30(22):7575-86. doi: 10.1523/JNEUROSCI.1312-10.2010. J Neurosci. 2010. PMID: 20519532 Free PMC article.
-
Protein kinase C potentiation of N-methyl-D-aspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits.Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):15262-7. doi: 10.1073/pnas.96.26.15262. Proc Natl Acad Sci U S A. 1999. PMID: 10611373 Free PMC article.
-
Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin.J Neurosci. 2001 Jan 15;21(2):423-33. doi: 10.1523/JNEUROSCI.21-02-00423.2001. J Neurosci. 2001. PMID: 11160423 Free PMC article.
-
Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors.J Neurosci. 2003 Nov 5;23(31):10064-73. doi: 10.1523/JNEUROSCI.23-31-10064.2003. J Neurosci. 2003. PMID: 14602821 Free PMC article.
References
-
- Baron MD, Davison MD, Jones P, Critchley DR. The sequence of chick α-actinin reveals homologies to spectrin and calmodulin. J Biol Chem. 1987;262:17623–17629. - PubMed
-
- Beggs AH, Byers TJ, Knoll JHM, Boyce FM, Bruns GAP, Kunkel LM. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. - PubMed
-
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–1065. - PubMed
-
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
