Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons
- PMID: 9952402
- PMCID: PMC6786014
- DOI: 10.1523/JNEUROSCI.19-04-01247.1999
Activation of protein kinase A contributes to the expression but not the induction of long-term hyperexcitability caused by axotomy of Aplysia sensory neurons
Abstract
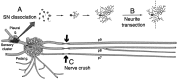
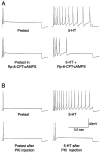
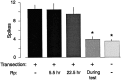
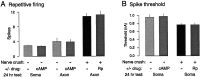
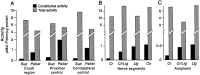
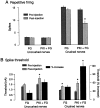
Nociceptive sensory neurons (SNs) in Aplysia provide useful models to study both memory and adaptive responses to nerve injury. Induction of long-term memory in many species, including Aplysia, is thought to depend on activation of cAMP-dependent protein kinase (PKA). Because Aplysia SNs display similar alterations in models of memory and after nerve injury, a plausible hypothesis is that axotomy triggers memory-like modifications by activating PKA in damaged axons. The present study disproves this hypothesis. SN axotomy was produced by (1) dissociation of somata from the ganglion [which is shown to induce long-term hyperexcitability (LTH)], (2) transection of neurites of dissociated SNs growing in vitro, or (3) peripheral nerve crush. Application of the competitive PKA inhibitor Rp-8-CPT-cAMPS at the time of axotomy failed to alter the induction of LTH by each form of axotomy, although the inhibitor antagonized hyperexcitability produced by 5-HT application. Strong activation of PKA in the nerve by coapplication of a membrane-permeant analog of cAMP and a phosphodiesterase inhibitor was not sufficient to induce LTH of either the SN somata or axons. Furthermore, nerve crush failed to activate axonal PKA or stimulate its retrograde transport. Therefore, PKA activation plays little if any role in the induction of LTH by axotomy. However, the expression of LTH was reduced by intracellular injection of the highly specific PKA inhibitor PKI several days after nerve crush. This suggests that long-lasting activation of PKA in or near the soma contributes to the maintenance of long-term modifications produced by nerve injury.
Figures


References
-
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–378. - PubMed
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. - PubMed
-
- Ambron RT, Walters ET. Priming events and retrograde injury signals: a new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
