Ciliary neurotrophic factor is a regulator of muscular strength in aging
- PMID: 9952403
- PMCID: PMC6786012
- DOI: 10.1523/JNEUROSCI.19-04-01257.1999
Ciliary neurotrophic factor is a regulator of muscular strength in aging
Abstract
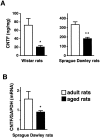
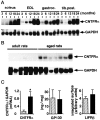
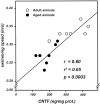
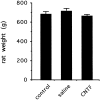
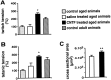
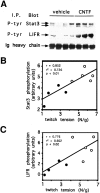
Ciliary neurotrophic factor (CNTF) participates in the survival of motor neurons and reduces the denervation-induced atrophy of skeletal muscles. Experiments performed in rats show a decrease in peripheral CNTF synthesis during aging, associated with an overexpression of its alpha-binding receptor component by skeletal muscles. Measurement of sciatic nerve CNTF production and of the muscular performance developed by the animals revealed a strong correlation between the two studied parameters (r = 0.8; p < 0.0003). Furthermore, the twitch and tetanic tensions measured in the isolated soleus skeletal muscle in 24-month-old animals increased 2. 5-fold by continuous in vivo administration of CNTF. Analyses of the activation level of leukemia inhibitory factor receptor beta- and signal transducer and activator of transcription 3-signaling molecules in response to exogenous CNTF revealed an increased tyrosine phosphorylation positively correlated with the twitch tension developed by the soleus muscle of the animals.
Figures
References
-
- Apfel SC, Arezzo JC, Moran M, Kessler JA. Effects of administration of ciliary neurotrophic factor on normal motor and sensory peripheral nerves in vivo. Brain Res. 1993;604:1–6. - PubMed
-
- Chevalier S, Fourcin M, Robledo O, Wijdenes J, Pouplard-Barthelaix A, Gascan H. Interleukin-6 family of cytokines induced activation of different functional sites expressed by gp130 transducing protein. J Biol Chem. 1996;271:14764–14772. - PubMed
-
- Curtis R, Adryan KM, Zhu Y, Harkness PJ, Lindsay RM, DiStefano PS. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature. 1993;365:253–255. - PubMed
-
- Davis S, Aldrich TH, Valenzuela DM, Wong V, Furth ME, Squinto SP, Yancopoulos GD. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. - PubMed
-
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFRβ and gp130 as hetero-dimerizing signal transducers of the tripartite CNTF receptor. Science. 1993a;260:1805–1808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
