Mutation in neurofilament transgene implicates RNA processing in the pathogenesis of neurodegenerative disease
- PMID: 9952405
- PMCID: PMC6786029
- DOI: 10.1523/JNEUROSCI.19-04-01273.1999
Mutation in neurofilament transgene implicates RNA processing in the pathogenesis of neurodegenerative disease
Abstract
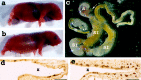
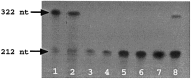
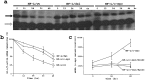
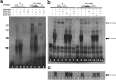
A mouse neurofilament light subunit (NF-L) transgene with a 36 bp c-myc insert at the end of the coding region was found to have neuropathic effects on enteric and motor neurons of transgenic mice. The severity of phenotype was related directly to the levels of transgenic mRNA expression. High levels of transgene expression were lethal to newborn pups, causing profound alterations in the development of the enteric nervous system and extensive vacuolar changes in motor neurons. Lower levels of transgene expression led to a transient stunting of growth and focal alterations of enteric and motor neurons. Because the positioning of the c-myc insert coincided with the location of the major stability determinant of the NF-L mRNA (Cañete-Soler et al., 1998a,b), additional studies were undertaken. These studies showed that the c-myc insert alters the ribonucleoprotein (RNP) complexes that bind to the stability determinant and disrupts their ability to regulate the stability of the transcripts. The findings indicate that expression of an NF-L transgene with a mutant mRNA stability determinant is highly disruptive to enteric and motor neurons and implicate alterations in RNA processing in the pathogenesis of a neurodegenerative condition.
Figures



Similar articles
-
3' untranslated region in a light neurofilament (NF-L) mRNA triggers aggregation of NF-L and mutant superoxide dismutase 1 proteins in neuronal cells.J Neurosci. 2004 Mar 17;24(11):2716-26. doi: 10.1523/JNEUROSCI.5689-03.2004. J Neurosci. 2004. PMID: 15028764 Free PMC article.
-
Untranslated element in neurofilament mRNA has neuropathic effect on motor neurons of transgenic mice.J Neurosci. 2002 Sep 1;22(17):7662-70. doi: 10.1523/JNEUROSCI.22-17-07662.2002. J Neurosci. 2002. PMID: 12196589 Free PMC article.
-
Post-transcriptional control of neurofilaments in development and disease.Exp Cell Res. 2007 Jun 10;313(10):2088-97. doi: 10.1016/j.yexcr.2007.02.014. Epub 2007 Feb 27. Exp Cell Res. 2007. PMID: 17428473 Review.
-
Similar poly(C)-sensitive RNA-binding complexes regulate the stability of the heavy and light neurofilament mRNAs.Brain Res. 2000 Jun 9;867(1-2):265-79. doi: 10.1016/s0006-8993(00)02389-1. Brain Res. 2000. PMID: 10837825
-
Post-transcriptional control of neurofilaments: New roles in development, regeneration and neurodegenerative disease.Trends Neurosci. 2010 Jan;33(1):27-37. doi: 10.1016/j.tins.2009.10.002. Epub 2009 Nov 10. Trends Neurosci. 2010. PMID: 19906448 Review.
Cited by
-
3' untranslated region in a light neurofilament (NF-L) mRNA triggers aggregation of NF-L and mutant superoxide dismutase 1 proteins in neuronal cells.J Neurosci. 2004 Mar 17;24(11):2716-26. doi: 10.1523/JNEUROSCI.5689-03.2004. J Neurosci. 2004. PMID: 15028764 Free PMC article.
-
miRNA malfunction causes spinal motor neuron disease.Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13111-6. doi: 10.1073/pnas.1006151107. Epub 2010 Jun 29. Proc Natl Acad Sci U S A. 2010. PMID: 20616011 Free PMC article.
-
Overexpression of the 3'-untranslated region of myelin proteolipid protein mRNA leads to reduced expression of endogenous proteolipid mRNA.Neurochem Res. 2002 Nov;27(11):1349-60. doi: 10.1023/a:1021623700009. Neurochem Res. 2002. PMID: 12512940
-
Aldolases a and C are ribonucleolytic components of a neuronal complex that regulates the stability of the light-neurofilament mRNA.J Neurosci. 2005 Apr 27;25(17):4353-64. doi: 10.1523/JNEUROSCI.0885-05.2005. J Neurosci. 2005. PMID: 15858061 Free PMC article.
-
The cytoskeleton in neurodegenerative diseases.J Pathol. 2004 Nov;204(4):438-49. doi: 10.1002/path.1650. J Pathol. 2004. PMID: 15495240 Free PMC article. Review.
References
-
- Antic D, Keene JD. Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J Cell Sci. 1998;111:183–197. - PubMed
-
- Bruijn LI, Cleveland DW. Mechanisms of selective motor neuron death in ALS: insights from transgenic mouse models of motor neuron disease. Neuropathol Appl Neurobiol. 1996;22:373–387. - PubMed
-
- Cañete-Soler R, Schwartz ML, Hua Y, Schlaepfer WW. Stability determinants are localized to the 3′UTR and 3′ coding region of the neurofilament light subunit mRNA using a tetracycline-inducible promoter. J Biol Chem. 1998a;273:12650–12654. - PubMed
-
- Cañete-Soler R, Schwartz ML, Hua Y, Schlaepfer WW. Characterization of ribonucleoprotein complexes and their binding sites on the neurofilament light subunit (NF-L) mRNA. J Biol Chem. 1998b;273:12655–12661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
