Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons
- PMID: 9952406
- PMCID: PMC6786015
- DOI: 10.1523/JNEUROSCI.19-04-01284.1999
Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons
Abstract
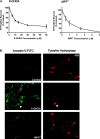
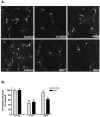
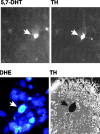
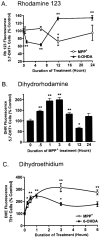
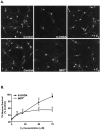
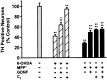
Oxidative stress is thought to contribute to dopaminergic cell death in Parkinson's disease (PD). The neurotoxin 6-hydroxydopamine (6-OHDA), which is easily oxidized to reactive oxygen species (ROS), appears to induce neuronal death by a free radical-mediated mechanism, whereas the involvement of free radicals in N-methyl-4-phenylpyridinium (MPP+) toxicity is less clear. Using free radical-sensitive fluorophores and vital dyes with post hoc identification of tyrosine hydroxylase-positive neurons, we monitored markers of apoptosis and the production of ROS in dopaminergic neurons treated with either 6-OHDA or MPP+. Annexin-V staining suggested that 6-OHDA but not MPP+-mediated cell death was apoptotic. In accordance with this assignment, the general caspase inhibitor Boc-(Asp)-fluoromethylketone only blocked 6-OHDA neurotoxicity. Both toxins exhibited an early, sustained rise in ROS, although only 6-OHDA induced a collapse in mitochondrial membrane potential temporally related to the increase in ROS. Recently, derivatives of buckminsterfullerene (C60) molecules have been shown to act as potent antioxidants in several models of oxidative stress (Dugan et al., 1997). Significant, dose-dependent levels of protection were also seen in these in vitro models of PD using the C3 carboxyfullerene derivative. Specifically, C3 was fully protective in the 6-OHDA paradigm, whereas it only partially rescued dopaminergic neurons from MPP+-induced cell death. In either model, it was more effective than glial-derived neurotrophic factor. These data suggest that cell death in response to 6-OHDA and MPP+ may progress through different mechanisms, which can be partially or entirely saved by carboxyfullerenes.
Figures
References
-
- Cardozo DL. Midbrain dopaminergic neurons from postnatal rat in long-term primary culture. Neuroscience. 1993;56:409–421. - PubMed
-
- Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J Neurophysiol. 1995;74:1137–1148. - PubMed
-
- Cassarino DS, Fall CP, Smith TS, Bennett JP., Jr Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J Neurochem. 1998;71:295–301. - PubMed
-
- Cohen G, Heikkila RE. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974;249:2447–2452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
