Molecular analysis of the X11-mLin-2/CASK complex in brain
- PMID: 9952408
- PMCID: PMC6786035
- DOI: 10.1523/JNEUROSCI.19-04-01307.1999
Molecular analysis of the X11-mLin-2/CASK complex in brain
Abstract
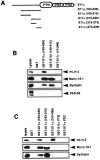
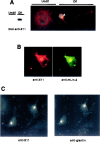
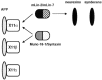
A heterotrimeric complex containing Lin-10/X11alpha, Lin-2/CASK, and Lin-7 is evolutionarily conserved from worms to mammals. In Caenorhabditis elegans, it localizes Let-23, a receptor tyrosine kinase, to the basolateral side of vulval epithelium, a step crucial for proper vulva development. In mammals, the complex may also participate in receptor targeting in neurons. Accordingly, phosphotyrosine binding (PTB) and postsynaptic density-95/Discs large/Zona Occludens-1 domains found in X11alpha and mLin-2/CASK bind to cell-surface proteins, including amyloid precursor protein, neurexins, and syndecans. In this paper, we have further analyzed the X11alpha-mLin-2/CASK association that is mediated by a novel protein-protein interaction. We show that the mLin-2/CASK calmodulin kinase II (CKII) domain directly binds to a 63 amino acids peptide located between the Munc-18-1 binding site and the PTB domain in X11alpha. Ca2+/calmodulin association with mLin-2/CASK does not modify the X11alpha-mLin-2 interaction. A region containing the mLin-2/CASK guanylate kinase domain also interacts with X11alpha but with a lower affinity than the CKII domain. Immunostaining of X11alpha in the brain shows that the protein is expressed in areas shown previously to be positive for mLin-2/CASK staining. Together, our data demonstrate that the X11alpha-mLin-2 complex contacts many partners, creating a macrocomplex suitable for receptor targeting at the neuronal plasma membrane.
Figures






References
-
- Borg J-P, Margolis B. Function of PTB domains. Curr Top Microbiol Immunol. 1998;228:23–38. - PubMed
-
- Borg J-P, Straight SW, Kaech SM, De Taddeo-Borg M, Kroon DE, Karnak D, Turner RS, Kim SK, Margolis B. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J Biol Chem. 1998a;273:31633–31636. - PubMed
-
- Borg J-P, Yang Y, De Taddeo-Borg M, Margolis B, Turner RS. The X11α protein slows cellular amyloid precursor protein processing and reduces Aβ40 and Aβ42 secretion. J Biol Chem. 1998b;273:14761–14766. - PubMed
-
- Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
