Organization of disparity-selective neurons in macaque area MT
- PMID: 9952417
- PMCID: PMC6786027
- DOI: 10.1523/JNEUROSCI.19-04-01398.1999
Organization of disparity-selective neurons in macaque area MT
Abstract
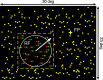
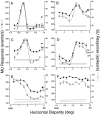
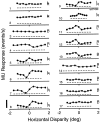
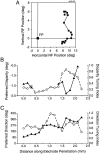
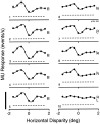
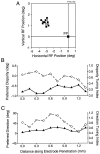
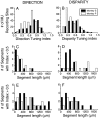
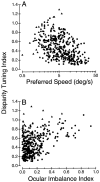
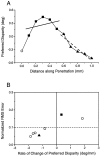
Neurons selective for binocular disparity are found in a number of visual cortical areas in primates, but there is little evidence that any of these areas are specialized for disparity processing. We have examined the organization of disparity-selective neurons in the middle temporal visual area (MT), an area shown previously to contain an abundance of disparity-sensitive neurons. We recorded extracellularly from MT neurons at regularly spaced intervals along electrode penetrations that passed through MT either normal to the cortical surface or at a shallow oblique angle. Comparison of multiunit and single-unit recordings shows that neurons are clustered in MT according to their disparity selectivity. Across the surface of MT, disparity-selective neurons are found in discrete patches that are separated by regions of MT that exhibit poor disparity tuning. Within disparity-selective patches of MT, we typically observe a smooth progression of preferred disparities (e.g. , near to far) as our electrode travels parallel to the cortical surface. In electrode penetrations normal to the cortical surface, on the other hand, MT neurons generally have similar disparity tuning, with little variation from one recording site to the next. Thus disparity-tuned neurons are organized into cortical columns by preferred disparity, and preferred disparity is mapped systematically within larger, disparity-tuned patches of MT. Combined with other recent findings, the data suggest that MT plays an important role in stereoscopic depth perception in addition to its well known role in motion perception.
Figures









References
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. - PubMed
-
- Albright TD. Cortical processing of visual motion. Rev Oculomotor Res. 1993;5:177–201. - PubMed
-
- Albright TD, Desimone R. Local precision of visuotopic organization in the middle temporal area (MT) of the macaque. Exp Brain Res. 1987;65:582–592. - PubMed
-
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984;51:16–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
