Structure, properties, and tissue localization of apoplastic alpha-glucosidase in crucifers
- PMID: 9952433
- PMCID: PMC32114
- DOI: 10.1104/pp.119.2.385
Structure, properties, and tissue localization of apoplastic alpha-glucosidase in crucifers
Abstract
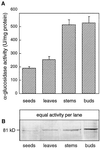
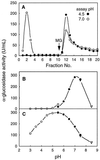
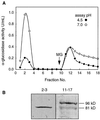
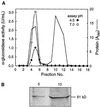
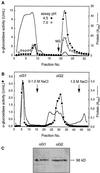
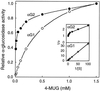
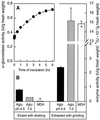
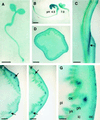
Apoplastic alpha-glucosidases occur widely in plants but their function is unknown because appropriate substrates in the apoplast have not been identified. Arabidopsis contains at least three alpha-glucosidase genes; Aglu-1 and Aglu-3 are sequenced and Aglu-2 is known from six expressed sequence tags. Antibodies raised to a portion of Aglu-1 expressed in Escherichia coli recognize two proteins of 96 and 81 kD, respectively, in vegetative tissues of Arabidopsis, broccoli (Brassica oleracea L.), and mustard (Brassica napus L.). The acidic alpha-glucosidase activity from broccoli flower buds was purified using concanavalin A and ion-exchange chromatography. Two active fractions were resolved and both contained a 96-kD immunoreactive polypeptide. The N-terminal sequence from the 96-kD broccoli alpha-glucosidase indicated that it corresponds to the Arabidopsis Aglu-2 gene and that approximately 15 kD of the predicted N terminus was cleaved. The 81-kD protein was more abundant than the 96-kD protein, but it was not active with 4-methylumbelliferyl-alpha-D-glucopyranoside as the substrate and it did not bind to concanavalin A. In situ activity staining using 5-bromo-4-chloro-3-indolyl-alpha-D-glucopyranoside revealed that the acidic alpha-glucosidase activity is predominantly located in the outer cortex of broccoli stems and in vascular tissue, especially in leaf traces.
Figures



References
-
- Arendt CW, Ostergaard HL. Identification of the CD45-associated 116-kDa and 80-kDa proteins as the alpha- and beta-subunits of alpha-glucosidase II. J Biol Chem. 1997;272:13117–13125. - PubMed
-
- Beckerman JL, Ebbole DJ. MPG1, a gene encoding a fungal hydrophobin of Magnaporthe grisea, is involved in surface recognition. Mol Plant Microbe Interact. 1996;9:450–456. - PubMed
-
- Chiba S, Inomata S, Matsui H, Shimomura T. Purification and properties of an α-glucosidase (glucoamylase) in sugar beet seed. Agric Biol Chem. 1978;42:241–245.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

