Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon
- PMID: 9952436
- PMCID: PMC32117
- DOI: 10.1104/pp.119.2.417
Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon
Abstract
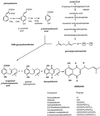
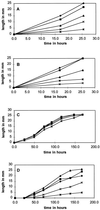
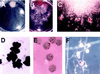
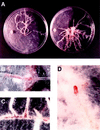
Pigmented naphthoquinone derivatives of shikonin are produced at specific times and in specific cells of Lithospermum erythrorhizon roots. Normal pigment development is limited to root hairs and root border cells in hairy roots grown on "noninducing" medium, whereas induction of additional pigment production by abiotic (CuSO4) or biotic (fungal elicitor) factors increases the amount of total pigment, changes the ratios of derivatives produced, and initiates production of pigment de novo in epidermal cells. When the biological activity of these compounds was tested against soil-borne bacteria and fungi, a wide range of sensitivity was recorded. Acetyl-shikonin and beta-hydroxyisovaleryl-shikonin, the two most abundant derivatives in both Agrobacterium rhizogenes-transformed "hairy-root" cultures and greenhouse-grown plant roots, were the most biologically active of the seven compounds tested. Hyphae of the pathogenic fungi Rhizoctonia solani, Pythium aphanidermatum, and Nectria hematococca induced localized pigment production upon contact with the roots. Challenge by R. solani crude elicitor increased shikonin derivative production 30-fold. We have studied the regulation of this suite of related, differentially produced, differentially active compounds to understand their role(s) in plant defense at the cellular level in the rhizosphere.
Figures

Similar articles
-
Stable transformation of Lithospermum erythrorhizon by Agrobacterium rhizogenes and shikonin production of the transformants.Plant Cell Rep. 1998 Dec;18(3-4):214-219. doi: 10.1007/s002990050559. Plant Cell Rep. 1998. PMID: 30744223
-
Shikonin production by extractive cultivation in transformed-suspension and hairy root cultures of Lithospermum erythrorhizon.Ann N Y Acad Sci. 1994 Nov 30;745:442-54. doi: 10.1111/j.1749-6632.1994.tb44395.x. Ann N Y Acad Sci. 1994. PMID: 7832530
-
Characterization of Shikonin Derivative Secretion in Lithospermum erythrorhizon Hairy Roots as a Model of Lipid-Soluble Metabolite Secretion from Plants.Front Plant Sci. 2016 Jul 26;7:1066. doi: 10.3389/fpls.2016.01066. eCollection 2016. Front Plant Sci. 2016. PMID: 27507975 Free PMC article.
-
Lithospermum erythrorhizon cell cultures: Present and future aspects.Plant Biotechnol (Tokyo). 2017;34(3):131-142. doi: 10.5511/plantbiotechnology.17.0823a. Epub 2017 Sep 27. Plant Biotechnol (Tokyo). 2017. PMID: 31275019 Free PMC article. Review.
-
Hairy Root Cultures for the Production of Anti-cancer Naphthoquinone Compounds.Curr Med Chem. 2018;25(36):4718-4739. doi: 10.2174/0929867324666170821161844. Curr Med Chem. 2018. PMID: 28828978 Review.
Cited by
-
Regulation of monoterpene accumulation in leaves of peppermint.Plant Physiol. 2000 Jan;122(1):205-14. doi: 10.1104/pp.122.1.205. Plant Physiol. 2000. PMID: 10631264 Free PMC article.
-
Shikonin Promotes Apoptosis and Attenuates Migration and Invasion of Human Esophageal Cancer Cells by Inhibiting Tumor Necrosis Factor Receptor-Associated Protein 1 Expression and AKT/mTOR Signaling Pathway.Evid Based Complement Alternat Med. 2021 Nov 13;2021:5386050. doi: 10.1155/2021/5386050. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34812264 Free PMC article.
-
Deoxyshikonin: a promising lead drug grass against drug resistance or sensitivity to Helicobacter pylori in an acidic environment.Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0095924. doi: 10.1128/aac.00959-24. Epub 2024 Aug 22. Antimicrob Agents Chemother. 2024. PMID: 39171918 Free PMC article.
-
Root exudates enhanced rhizobacteria complexity and microbial carbon metabolism of toxic plants.iScience. 2022 Sep 29;25(10):105243. doi: 10.1016/j.isci.2022.105243. eCollection 2022 Oct 21. iScience. 2022. PMID: 36274956 Free PMC article.
-
Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica.Front Plant Sci. 2014 Feb 11;5:27. doi: 10.3389/fpls.2014.00027. eCollection 2014. Front Plant Sci. 2014. PMID: 24575103 Free PMC article. Review.
References
-
- Bago B, Vierheilig H, Piché Y, Azcón-Aguilar C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol. 1996;133:273–280. - PubMed
-
- Ballantine JA. The isolation of two esters of the naphthoquinone alcohol, shikonin, from the shrub Jatropha glandulifera. Phytochemistry. 1969;8:1587–1590.
-
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. - PubMed
-
- Bennett RN, Wallsgrove RM. Secondary metabolites in plant defense mechanisms. New Phytol. 1994;127:617–633. - PubMed
-
- Binder RG, Benson ME, Flath RA. Eight 1,4-naphthoquinones from Juglans. Phytochemistry. 1989;28:2799–2801.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

