Rhizosphere bacteria enhance selenium accumulation and volatilization by indian mustard
- PMID: 9952452
- PMCID: PMC32133
- DOI: 10.1104/pp.119.2.565
Rhizosphere bacteria enhance selenium accumulation and volatilization by indian mustard
Abstract
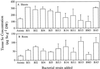
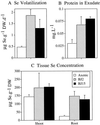
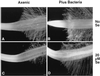
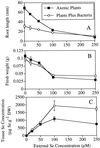
Indian mustard (Brassica juncea L.) accumulates high tissue Se concentrations and volatilizes Se in relatively nontoxic forms, such as dimethylselenide. This study showed that the presence of bacteria in the rhizosphere of Indian mustard was necessary to achieve the best rates of plant Se accumulation and volatilization of selenate. Experiments with the antibiotic ampicillin showed that bacteria facilitated 35% of plant Se volatilization and 70% of plant tissue accumulation. These results were confirmed by inoculating axenic plants with rhizosphere bacteria. Compared with axenic controls, plants inoculated with rhizosphere bacteria had 5-fold higher Se concentrations in roots (the site of volatilization) and 4-fold higher rates of Se volatilization. Plants with bacteria contained a heat-labile compound in their root exudate; when this compound was added to the rhizosphere of axenic plants, Se accumulation in plant tissues increased. Plants with bacteria had an increased root surface area compared with axenic plants; the increased area was unlikely to have caused their increased tissue Se accumulation because they did not accumulate more Se when supplied with selenite or selenomethionine. Rhizosphere bacteria also possibly increased plant Se volatilization because they enabled plants to overcome a rate-limiting step in the Se volatilization pathway, i.e. Se accumulation in plant tissues.
Figures



References
-
- Abrams MM, Shennan C, Zasoski RJ, Burau RG. Selenomethionine uptake by wheat seedlings. Agron J. 1990;82:1127–1130.
-
- Anderson TA, Guthrie EA, Walton BT. Bioremediation in the rhizosphere: plant roots and associated microbes clean contaminated soil. Environ Sci Technol. 1993;27:2630–2636.
-
- Arvy MP. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris) J Exp Bot. 1993;44:1083–1087.
-
- Atkinson R, Aschmann SM, Hasegawa D, Thompson-Eagle ET, Frankenberger WT., Jr Kinetics of the atmospherically important reactions of dimethyl selenide. Environ Sci Technol. 1990;24:1326–1332.
-
- Aubert H, Pinta M (1977) Trace Elements in Soils. Elsevier Science Publishers, New York, pp 5–11
LinkOut - more resources
Full Text Sources
Other Literature Sources

