Red bell pepper chromoplasts exhibit in vitro import competency and membrane targeting of passenger proteins from the thylakoidal sec and DeltapH pathways but not the chloroplast signal recognition particle pathway
- PMID: 9952453
- PMCID: PMC32134
- DOI: 10.1104/pp.119.2.575
Red bell pepper chromoplasts exhibit in vitro import competency and membrane targeting of passenger proteins from the thylakoidal sec and DeltapH pathways but not the chloroplast signal recognition particle pathway
Abstract
Chloroplast to chromoplast development involves new synthesis and plastid localization of nuclear-encoded proteins, as well as changes in the organization of internal plastid membrane compartments. We have demonstrated that isolated red bell pepper (Capsicum annuum) chromoplasts contain the 75-kD component of the chloroplast outer envelope translocon (Toc75) and are capable of importing chloroplast precursors in an ATP-dependent fashion, indicating a functional general import apparatus. The isolated chromoplasts were able to further localize the 33- and 17-kD subunits of the photosystem II O2-evolution complex (OE33 and OE17, respectively), lumen-targeted precursors that utilize the thylakoidal Sec and DeltapH pathways, respectively, to the lumen of an internal membrane compartment. Chromoplasts contained the thylakoid Sec component protein, cpSecA, at levels comparable to chloroplasts. Routing of OE17 to the lumen was abolished by ionophores, suggesting that routing is dependent on a transmembrane DeltapH. The chloroplast signal recognition particle pathway precursor major photosystem II light-harvesting chlorophyll a/b protein failed to associate with chromoplast membranes and instead accumulated in the stroma following import. The Pftf (plastid fusion/translocation factor), a chromoplast protein, integrated into the internal membranes of chromoplasts during in vitro assays, and immunoblot analysis indicated that endogenous plastid fusion/translocation factor was also an integral membrane protein of chromoplasts. These data demonstrate that the internal membranes of chromoplasts are functional with respect to protein translocation on the thylakoid Sec and DeltapH pathways.
Figures

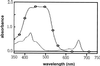
 , Red bell
pepper chromoplast extract; ———, green pea chloroplast extract.
A652 of chromoplasts = 0.012;
A652 of chloroplasts = 0.256
, Red bell
pepper chromoplast extract; ———, green pea chloroplast extract.
A652 of chromoplasts = 0.012;
A652 of chloroplasts = 0.256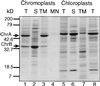




Similar articles
-
Identification of a plastid protein involved in vesicle fusion and/or membrane protein translocation.Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5630-4. doi: 10.1073/pnas.92.12.5630. Proc Natl Acad Sci U S A. 1995. PMID: 7777561 Free PMC article.
-
Proteome analysis of bell pepper (Capsicum annuum L.) chromoplasts.Plant Cell Physiol. 2006 Dec;47(12):1663-73. doi: 10.1093/pcp/pcl033. Epub 2006 Nov 10. Plant Cell Physiol. 2006. PMID: 17098784
-
Differentiation of chromoplasts and other plastids in plants.Plant Cell Rep. 2019 Jul;38(7):803-818. doi: 10.1007/s00299-019-02420-2. Epub 2019 May 11. Plant Cell Rep. 2019. PMID: 31079194 Free PMC article. Review.
-
Proteomic analysis of chromoplasts from six crop species reveals insights into chromoplast function and development.J Exp Bot. 2013 Feb;64(4):949-61. doi: 10.1093/jxb/ers375. Epub 2013 Jan 10. J Exp Bot. 2013. PMID: 23314817 Free PMC article.
-
Recognition and envelope translocation of chloroplast preproteins.J Exp Bot. 2005 Sep;56(419):2287-320. doi: 10.1093/jxb/eri243. Epub 2005 Aug 8. J Exp Bot. 2005. PMID: 16087701 Review.
Cited by
-
Two chloroplastic protein translocation components, Tic110 and Toc75, are conserved in different plastid types from multiple plant species.Plant Mol Biol. 2003 Jan;51(2):175-81. doi: 10.1023/a:1021190319786. Plant Mol Biol. 2003. PMID: 12602876
-
Tomato fruit chromoplasts behave as respiratory bioenergetic organelles during ripening.Plant Physiol. 2014 Oct;166(2):920-33. doi: 10.1104/pp.114.243931. Epub 2014 Aug 14. Plant Physiol. 2014. PMID: 25125503 Free PMC article.
-
In vivo analyses of the roles of essential Omp85-related proteins in the chloroplast outer envelope membrane.Plant Physiol. 2011 Sep;157(1):147-59. doi: 10.1104/pp.111.181891. Epub 2011 Jul 14. Plant Physiol. 2011. PMID: 21757633 Free PMC article.
-
Molecular characterization and expression analysis of chloroplast protein import components in tomato (Solanum lycopersicum).PLoS One. 2014 Apr 21;9(4):e95088. doi: 10.1371/journal.pone.0095088. eCollection 2014. PLoS One. 2014. PMID: 24751891 Free PMC article.
-
A molecular-genetic study of the Arabidopsis Toc75 gene family.Plant Physiol. 2005 Jun;138(2):715-33. doi: 10.1104/pp.105.063289. Epub 2005 May 20. Plant Physiol. 2005. PMID: 15908591 Free PMC article.
References
-
- Bouvier F, Hugueney P, D'Harlingue A, Kuntz M, Camara B. Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J. 1994;6:45–54. - PubMed
-
- Camara B, Brangeon J. Carotenoid metabolism during chloroplast to chromoplast transformation in Capsicum annuum fruit. Planta. 1981;151:359–364. - PubMed
-
- Camara B, Hugueney P, Bouvier F, Kuntz M, Moneger R. Biochemistry and molecular biology of chromoplast development. Int Rev Cytol. 1995;163:175–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

