Flavonoids promote haustoria formation in the root parasite triphysaria versicolor
- PMID: 9952454
- PMCID: PMC32135
- DOI: 10.1104/pp.119.2.585
Flavonoids promote haustoria formation in the root parasite triphysaria versicolor
Abstract
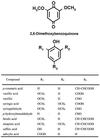
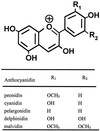
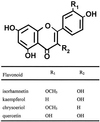
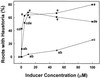
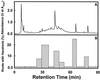
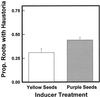
Parasitic plants in the Scrophulariaceae develop infective root structures called haustoria in response to chemical signals released from host-plant roots. This study used a simple in vitro assay to characterize natural and synthetic molecules that induce haustoria in the facultative parasite Triphysaria versicolor. Several phenolic acids, flavonoids, and the quinone 2,6-dimethoxy-p-benzoquinone induced haustoria in T. versicolor root tips within hours after treatment. The concentration at which different molecules were active varied widely, the most active being 2, 6-dimethoxy-p-benzoquinone and the anthocyanidin peonidin. Maize (Zea mays) seeds are rich sources of molecules that induce T. versicolor haustoria in vitro, and chromatographic analyses indicated that the active molecules present in maize-seed rinses include anthocyanins, other flavonoids, and simple phenolics. The presence of different classes of inducing molecules in seed rinses was substantiated by the observation that maize kernels deficient in chalcone synthase, a key enzyme in flavonoid biosynthesis, released haustoria-inducing molecules, although at reduced levels compared with wild-type kernels. We discuss these results in light of existing models for host perception in the related parasitic plant Striga.
Figures


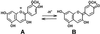
References
-
- Atsatt P, Strong D. The population biology of annual grassland hemiparasites. I. The host environment. Evolution. 1970;24:278–291. - PubMed
-
- Baird WV, Riopel JL. Experimental studies of haustorium initiation and early development in Agalinis purpurea (L.) Raf. (Scrophulariaceae) Am J Bot. 1984;71:803–814.
-
- Becard G, Taylor LP, Douds DD, Pfeffer PE, Doner LW. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. Mol Plant Microbe Interact. 1995;8:252–258.
-
- Chabot S, Bel-Rhlid R, Chenevert R, Piche Y. Hyphal growth promotion in vitro of the VA mycorrhizal fungus, Gigaspora margarita Becker & Hall, by the activity of structurally specific flavonoid compounds under CO2-enriched conditions. New Phytol. 1992;122:461–467. - PubMed
-
- Chang M, Lynn DG. The haustorium and host recognition in parasitic angiosperms. J Chem Ecol. 1986;12:561–579. - PubMed
LinkOut - more resources
Full Text Sources

