Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis
- PMID: 9952456
- PMCID: PMC32137
- DOI: 10.1104/pp.119.2.599
Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis
Abstract
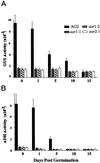
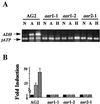
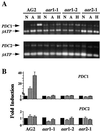
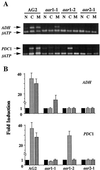
Expression of the alcohol dehydrogenase gene (ADH) of Arabidopsis is known to be induced by environmental stresses and regulated developmentally. We used a negative-selection approach to isolate mutants that were defective in regulating the expression of the ADH gene during seed germination; we then characterized three recessive mutants, aar1-1, aar1-2, and aar2-1, which belong to two complementation groups. In addition to their defects during seed germination, mutations in the AAR1 and AAR2 genes also affected anoxic and hypoxic induction of ADH and other glycolytic genes in mature plants. The aar1 and aar2 mutants were also defective in responding to cold and osmotic stress. The two allelic mutants aar1-1and aar1-2 exhibited different phenotypes under cold and osmotic stresses. Based on our results we propose that these mutants are defective in a late step of the signaling pathways that lead to increased expression of the ADH gene and glycolytic genes.
Figures


Similar articles
-
STRESS RESPONSE SUPPRESSOR1 and STRESS RESPONSE SUPPRESSOR2, two DEAD-box RNA helicases that attenuate Arabidopsis responses to multiple abiotic stresses.Plant Physiol. 2007 Nov;145(3):814-30. doi: 10.1104/pp.107.099895. Epub 2007 Jun 7. Plant Physiol. 2007. PMID: 17556511 Free PMC article.
-
Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene.Plant Physiol. 1994 Aug;105(4):1075-87. doi: 10.1104/pp.105.4.1075. Plant Physiol. 1994. PMID: 7972489 Free PMC article.
-
Crucial roles of the pentatricopeptide repeat protein SOAR1 in Arabidopsis response to drought, salt and cold stresses.Plant Mol Biol. 2015 Jul;88(4-5):369-85. doi: 10.1007/s11103-015-0327-9. Epub 2015 Jun 21. Plant Mol Biol. 2015. PMID: 26093896 Free PMC article.
-
Arabidopsis MADS-Box Transcription Factor AGL21 Acts as Environmental Surveillance of Seed Germination by Regulating ABI5 Expression.Mol Plant. 2017 Jun 5;10(6):834-845. doi: 10.1016/j.molp.2017.04.004. Epub 2017 Apr 22. Mol Plant. 2017. PMID: 28438576
-
A plant DNA ligase is an important determinant of seed longevity.Plant J. 2010 Sep;63(5):848-60. doi: 10.1111/j.1365-313X.2010.04285.x. Plant J. 2010. PMID: 20584150
Cited by
-
Mutations affecting light regulation of nuclear genes encoding chloroplast glyceraldehyde-3-phosphate dehydrogenase in Arabidopsis.Plant Physiol. 2002 Nov;130(3):1476-86. doi: 10.1104/pp.007849. Plant Physiol. 2002. PMID: 12428012 Free PMC article.
-
Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis.Plant Physiol. 2001 Jun;126(2):742-9. doi: 10.1104/pp.126.2.742. Plant Physiol. 2001. PMID: 11402202 Free PMC article.
-
Genome-wide identification of alcohol dehydrogenase (ADH) gene family in oilseed rape (Brassica napus L.) and BnADH36 functional verification under salt stress.BMC Plant Biol. 2024 Oct 28;24(1):1013. doi: 10.1186/s12870-024-05716-y. BMC Plant Biol. 2024. PMID: 39465389 Free PMC article.
-
The pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses.Plant Physiol. 2003 Jun;132(2):968-78. doi: 10.1104/pp.102.016907. Epub 2003 Apr 24. Plant Physiol. 2003. PMID: 12805625 Free PMC article.
-
Transgene expression patterns indicate that spaceflight affects stress signal perception and transduction in arabidopsis.Plant Physiol. 2001 Jun;126(2):613-21. doi: 10.1104/pp.126.2.613. Plant Physiol. 2001. PMID: 11402191 Free PMC article.
References
-
- Campbell R, Drew MC. Electron microscopy of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to oxygen shortage. Planta. 1983;157:350–357. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

