The wheat peptidyl prolyl cis-trans-isomerase FKBP77 is heat induced and developmentally regulated
- PMID: 9952466
- PMCID: PMC32147
- DOI: 10.1104/pp.119.2.693
The wheat peptidyl prolyl cis-trans-isomerase FKBP77 is heat induced and developmentally regulated
Abstract
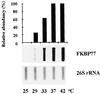
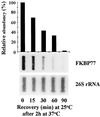
We isolated a cDNA encoding a 568-amino acid, heat-stress-induced peptidyl prolyl isomerase belonging to the FK506-binding-protein (FKBP) family. The open reading frame encodes for a peptidyl prolyl isomerase that possesses three FKBP-12-like domains, a putative tetratricopeptide motif, and a calmodulin-binding domain. Specific antibodies showed that the open reading frame encodes a heat-induced 77-kD protein, the wheat FKBP77 (wFKBP77), which exhibits 84% identity with the wFKBP73 and 42% identity with the human FKBP59. Because of the high similarity in sequence to wFKBP73, wFKBP77 was designated as the heat-induced isoform. The wFKBP77 mRNA steady-state level was 14-fold higher at 37 degreesC than at 25 degreesC. The wFKBP77 transcript abundance was the highest in mature embryos that had imbibed and 2-d-old green shoots exposed to 37 degreesC, and decreased to 6% in 6-d-old green shoots. The transcript level returned to the level detected at 25 degreesC after recovery of the embryos for 90 min at 25 degreesC. We compared wFKBP73 and wFKBP77 with the heat-shock proteins having cognate and heat-stress-induced counterparts.
Figures





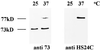


Similar articles
-
A novel plant peptidyl-prolyl-cis-trans-isomerase (PPIase): cDNA cloning, structural analysis, enzymatic activity and expression.Plant Mol Biol. 1996 Nov;32(3):493-504. doi: 10.1007/BF00019101. Plant Mol Biol. 1996. PMID: 8980498
-
Wheat FKBP73 functions in vitro as a molecular chaperone independently of its peptidyl prolyl cis-trans isomerase activity.Planta. 2002 May;215(1):119-26. doi: 10.1007/s00425-001-0722-0. Epub 2002 Feb 1. Planta. 2002. PMID: 12012248
-
Overexpression of the wheat FK506-binding protein 73 (FKBP73) and the heat-induced wheat FKBP77 in transgenic wheat reveals different functions of the two isoforms.Transgenic Res. 2002 Aug;11(4):373-9. doi: 10.1023/a:1016374128479. Transgenic Res. 2002. PMID: 12212840
-
Dual-Family Peptidylprolyl Isomerases (Immunophilins) of Select Monocellular Organisms.Biomolecules. 2018 Nov 15;8(4):148. doi: 10.3390/biom8040148. Biomolecules. 2018. PMID: 30445770 Free PMC article. Review.
-
Chlamydomonas immunophilins and parvulins: survey and critical assessment of gene models.Eukaryot Cell. 2005 Feb;4(2):230-41. doi: 10.1128/EC.4.2.230-241.2005. Eukaryot Cell. 2005. PMID: 15701785 Free PMC article. Review. No abstract available.
Cited by
-
Transcriptome-wide Identification of Nine Tandem Repeat Protein Families in Roselle (Hibiscus sabdariffa L.).Trop Life Sci Res. 2024 Oct;35(3):121-148. doi: 10.21315/tlsr2024.35.3.6. Epub 2024 Oct 7. Trop Life Sci Res. 2024. PMID: 39464663 Free PMC article.
-
Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress.BMC Plant Biol. 2010 Nov 18;10:253. doi: 10.1186/1471-2229-10-253. BMC Plant Biol. 2010. PMID: 21087465 Free PMC article.
-
SSH Analysis of Endosperm Transcripts and Characterization of Heat Stress Regulated Expressed Sequence Tags in Bread Wheat.Front Plant Sci. 2016 Aug 17;7:1230. doi: 10.3389/fpls.2016.01230. eCollection 2016. Front Plant Sci. 2016. PMID: 27582756 Free PMC article.
-
Identifying genetic variation associated with environmental gradients and drought-tolerance phenotypes in ponderosa pine.Ecol Evol. 2023 Oct 13;13(10):e10620. doi: 10.1002/ece3.10620. eCollection 2023 Oct. Ecol Evol. 2023. PMID: 37841219 Free PMC article.
-
Integration of meta-analysis, machine learning and systems biology approach for investigating the transcriptomic response to drought stress in Populus species.Sci Rep. 2023 Jan 16;13(1):847. doi: 10.1038/s41598-023-27746-6. Sci Rep. 2023. PMID: 36646724 Free PMC article.
References
-
- Bessudo A (1996) Molecular characterization of HSP80: a microtubule-associated protein in common wheat. Its gene copy number, location and expression. PhD thesis. Weizmann Institute of Science, Rehovot, Israel
-
- Blecher O, Erel N, Callebaut I, Aviezer K, Breiman A. A novel wheat peptidyl-prolyl cis trans isomerase: cDNA isolation, structure, enzymatic activity and expression. Plant Mol Biol. 1996;32:493–504. - PubMed
-
- Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of HSP90-associated proteins. Science. 1996;274:1715–1717. - PubMed
-
- Boston RS, Viitaner PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. - PubMed
-
- Breiman A, Fawcett TW, Ghirardi ML, Mattoo AK. Plant organelles contain distinct peptidyl-prolyl-cis-trans-isomerases. J Biol Chem. 1992;267:21293–21296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

