Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato
- PMID: 9952473
- PMCID: PMC32154
- DOI: 10.1104/pp.119.2.765
Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato
Abstract
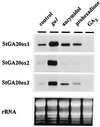
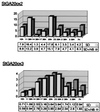
Tuber formation in potato (Solanum tuberosum) is promoted by short photoperiods and is inhibited by gibberellins (GAs). Endogenous levels of GA1 were shown to decrease in stolons and leaves of potato plants induced to tuberize, which suggests that photoperiodic regulation of GA biosynthesis may play a role in tuber induction. We report the isolation of three potato cDNA clones (StGA20ox1-3) encoding GA 20-oxidase, a key regulatory enzyme in the GA-biosynthetic pathway. Using northern analysis, we detected a differential pattern of tissue-specific expression of the mRNAs corresponding to these clones. StGA20ox mRNAs were also very abundant in leaves of the potato ga1 mutant, which is blocked in the 13-hydroxylation step, and were strongly down-regulated by gibberellic acid, suggesting a feedback regulation of these genes. In plants grown in short-day (inductive) conditions, levels of the StGA20ox transcripts in leaves fluctuated during a 24-h period, with a peak of accumulation observed about 4 h after the lights were turned off. Interruption of the night with a 30-min "night break" of light (noninductive conditions) did not have a marked effect on the levels of accumulation of the three GA 20-oxidase mRNAs during the day, but it induced a second peak of expression of StGA20ox1 and StGA20ox3 transcripts late in the night. This observation, together with the finding that StGA20ox1 mRNA is expressed at high levels in leaves, suggests that night-break induction of this gene might play a role in the control of tuberization by regulating endogenous levels of GAs in response to daylength conditions.
Figures




References
-
- Amasino RM. Acceleration of nucleic acid hybridization rate by polyethylenglycol. Anal Biochem. 1986;152:304–307. - PubMed
-
- Bamberg JB, Hanneman RE. Characterization of a new gibberellin related dwarfism locus in potato (Solanum tuberosum L.) Am Potato J. 1991;68:45–52.
-
- Braun HP, Emmerman H, Mentzel H, Schmitz UK. Primary structure and expression of a gene encoding the cytosolic ribosomal protein S4 from potato. Biochim Biophys Acta. 1994;1218:435–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

