Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells
- PMID: 9971738
- PMCID: PMC2132906
- DOI: 10.1083/jcb.144.3.427
Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells
Abstract
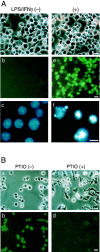
Excess nitric oxide (NO) induces apoptosis of some cell types, including macrophages. As NO is synthesized by NO synthase (NOS) from arginine, a common substrate of arginase, these two enzymes compete for arginine. There are two known isoforms of arginase, types I and II. Using murine macrophage-like RAW 264.7 cells, we asked if the induction of arginase II would downregulate NO production and hence prevent apoptosis. When cells were exposed to lipopolysaccharide (LPS) and interferon-gamma (IFN-gamma), the inducible form of NOS (iNOS) was induced, production of NO was elevated, and apoptosis followed. When dexamethasone and cAMP were further added, both iNOS and arginase II were induced, NO production was much decreased, and apoptosis was prevented. When the cells were transfected with an arginase II expression plasmid and treated with LPS/IFN-gamma, some cells were rescued from apoptosis. An arginase I expression plasmid was also effective. On the other hand, transfection with the arginase II plasmid did not prevent apoptosis when a NO donor SNAP or a high concentration (12 mM) of arginine was added. These results indicate that arginase II prevents NO-dependent apoptosis of RAW 264.7 cells by depleting intracellular arginine and by decreasing NO production.
Figures







Similar articles
-
Regulation of the urea cycle enzyme genes in nitric oxide synthesis.J Inherit Metab Dis. 1998;21 Suppl 1:59-71. doi: 10.1023/a:1005357608129. J Inherit Metab Dis. 1998. PMID: 9686345
-
Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells.Am J Physiol. 1998 Nov;275(5):E740-7. doi: 10.1152/ajpendo.1998.275.5.E740. Am J Physiol. 1998. PMID: 9814991
-
Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line.FEBS Lett. 1996 Oct 21;395(2-3):119-22. doi: 10.1016/0014-5793(96)01015-0. FEBS Lett. 1996. PMID: 8898077
-
Regulation of nitric oxide production by arginine metabolic enzymes.Biochem Biophys Res Commun. 2000 Sep 7;275(3):715-9. doi: 10.1006/bbrc.2000.3169. Biochem Biophys Res Commun. 2000. PMID: 10973788 Review.
-
Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling.J Nutr. 2007 Jun;137(6 Suppl 2):1616S-1620S. doi: 10.1093/jn/137.6.1616S. J Nutr. 2007. PMID: 17513437 Review.
Cited by
-
Interferon-gamma-induced nitric oxide inhibits the proliferation of murine renal cell carcinoma cells.Int J Biol Sci. 2012;8(8):1109-20. doi: 10.7150/ijbs.4694. Epub 2012 Sep 6. Int J Biol Sci. 2012. PMID: 22991499 Free PMC article.
-
Stimulation of phagocytic activity of alveolar macrophages toward artificial microspheres by infection with mycobacteria.Pharm Res. 2008 Jun;25(6):1420-30. doi: 10.1007/s11095-007-9525-8. Pharm Res. 2008. PMID: 18172577
-
Arginine and Arginases Modulate Metabolism, Tumor Microenvironment and Prostate Cancer Progression.Nutrients. 2021 Dec 16;13(12):4503. doi: 10.3390/nu13124503. Nutrients. 2021. PMID: 34960055 Free PMC article. Review.
-
Nitric oxide and related enzymes in asthma: relation to severity, enzyme function and inflammation.Clin Exp Allergy. 2012 May;42(5):760-8. doi: 10.1111/j.1365-2222.2011.03860.x. Epub 2011 Sep 25. Clin Exp Allergy. 2012. PMID: 22092728 Free PMC article.
-
Chemical characterization and in vitro immunomodulatory effects of different extracts of moss Hedwigia ciliata (Hedw.) P. Beauv. from the Vršačke Planine Mts., Serbia.PLoS One. 2021 Feb 11;16(2):e0246810. doi: 10.1371/journal.pone.0246810. eCollection 2021. PLoS One. 2021. PMID: 33571277 Free PMC article.
References
-
- Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/NO through a radical reaction. Biochemistry. 1993;32:827–832. - PubMed
-
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. - PubMed
-
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

