delta-catenin, an adhesive junction-associated protein which promotes cell scattering
- PMID: 9971746
- PMCID: PMC2132907
- DOI: 10.1083/jcb.144.3.519
delta-catenin, an adhesive junction-associated protein which promotes cell scattering
Abstract
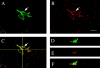
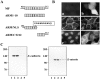
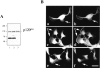
The classical adherens junction that holds epithelial cells together consists of a protein complex in which members of the cadherin family linked to various catenins are the principal components. delta-catenin is a mammalian brain protein in the Armadillo repeat superfamily with sequence similarity to the adherens junction protein p120(ctn). We found that delta-catenin can be immunoprecipitated as a complex with other components of the adherens junction, including cadherin and beta-catenin, from transfected cells and brain. The interaction with cadherin involves direct contact within the highly conserved juxtamembrane region of the COOH terminus, where p120(ctn) also binds. In developing mouse brain, staining with delta-catenin antibodies is prominent towards the apical boundary of the neuroepithelial cells in the ventricular zone. When transfected into Madin-Darby canine kidney (MDCK) epithelial cells delta-catenin colocalized with cadherin, p120(ctn), and beta-catenin. The Arm domain alone was sufficient for achieving localization and coimmunoprecipitation with cadherin. The ectopic expression of delta-catenin in MDCK cells altered their morphology, induced the elaboration of lamellipodia, interfered with monolayer formation, and increased scattering in response to hepatocyte growth factor treatment. We propose that delta-catenin can regulate adhesion molecules to implement the organization of large cellular arrays necessary for tissue morphogenesis.
Figures






References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
-
- Balkovetz DF, Pollack AL, Mostov KE. Hepatocyte growth factor alters the polarity of Madin-Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. - PubMed
-
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997a;9:683–690. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

