DE-Cadherin is required for intercellular motility during Drosophila oogenesis
- PMID: 9971747
- PMCID: PMC2132905
- DOI: 10.1083/jcb.144.3.533
DE-Cadherin is required for intercellular motility during Drosophila oogenesis
Abstract
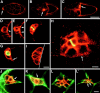
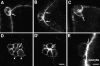
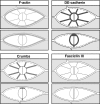
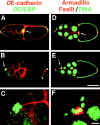
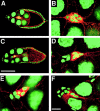
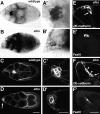
Cadherins are involved in a variety of morphogenetic movements during animal development. However, it has been difficult to pinpoint the precise function of cadherins in morphogenetic processes due to the multifunctional nature of cadherin requirement. The data presented here indicate that homophilic adhesion promoted by Drosophila E-cadherin (DE-cadherin) mediates two cell migration events during Drosophila oogenesis. In Drosophila follicles, two groups of follicle cells, the border cells and the centripetal cells migrate on the surface of germline cells. We show that the border cells migrate as an epithelial patch in which two centrally located cells retain epithelial polarity and peripheral cells are partially depolarized. Both follicle cells and germline cells express DE-cadherin, and border cells and centripetal cells strongly upregulate the expression of DE-cadherin shortly before and during their migration. Removing DE-cadherin from either the follicle cells or the germline cells blocks migration of border cells and centripetal cells on the surface of germline cells. The function of DE-cadherin in border cells appears to be specific for migration as the formation of the border cell cluster and the adhesion between border cells are not disrupted in the absence of DE-cadherin. The speed of migration depends on the level of DE-cadherin expression, as border cells migrate more slowly when DE-cadherin activity is reduced. Finally, we show that the upregulation of DE-cadherin expression in border cells depends on the activity of the Drosophila C/EBP transcription factor that is essential for border cell migration.
Figures




References
-
- Bellen HJ, O'Kane CJ, Wilson C, Grossniklaus U, Pearson RK, Gehring WJ. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. . Genes Dev. 1989;3:1288–1300. - PubMed
-
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. BioEssays. 1995;17:97–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

