Bacterial adhesins: common themes and variations in architecture and assembly
- PMID: 9973330
- PMCID: PMC93481
- DOI: 10.1128/JB.181.4.1059-1071.1999
Bacterial adhesins: common themes and variations in architecture and assembly
Figures

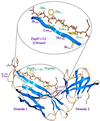




References
-
- Adams L M, Simmons C P, Rezmann L, Strugnell R A, Robins-Browne R M. Identification and characterization of a K88- and CS31A-like operon of a rabbit enteropathogenic Escherichia coli strain which encodes fimbriae involved in the colonization of rabbit intestine. Infect Immun. 1997;65:5222–5230. - PMC - PubMed
-
- Alm R A, Mattick J S. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1997;192:89–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

