Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces
- PMID: 9973338
- PMCID: PMC93489
- DOI: 10.1128/JB.181.4.1134-1140.1999
Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces
Abstract
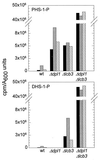
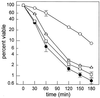
Sphingolipid long-chain bases and their phosphorylated derivatives, for example, sphingosine-1-phosphate in mammals, have been implicated as signaling molecules. The possibility that Saccharomyces cerevisiae cells also use long-chain-base phosphates to regulate cellular processes has only recently begun to be examined. Here we present a simple and sensitive procedure for analyzing and quantifying long-chain-base phosphates in S. cerevisiae cells. Our data show for the first time that phytosphingosine-1-phosphate (PHS-1-P) is present at a low but detectable level in cells grown on a fermentable carbon source at 25 degreesC, while dihydrosphingosine-1-phosphate (DHS-1-P) is only barely detectable. Shifting cells to 37 degreesC causes transient eight- and fivefold increases in levels of PHS-1-P and DHS-1-P, respectively, which peak after about 10 min. The amounts of both compounds return to the unstressed levels by 20 min after the temperature shift. These data are consistent with PHS-1-P and DHS-1-P being signaling molecules. Cells unable to break down long-chain-base phosphates, due to deletion of DPL1 and LCB3, show a 500-fold increase in PHS-1-P and DHS-1-P levels, grow slowly, and survive a 44 degreesC heat stress 10-fold better than parental cells. These and other data for dpl1 or lcb3 single-mutant strains suggest that DHS-1-P and/or PHS-1-P act as signals for resistance to heat stress. Our procedure should expedite experiments to determine how the synthesis and breakdown of these compounds is regulated and how the compounds mediate resistance to elevated temperature.
Figures




References
-
- Bornfeldt K E, Graves L M, Raines E W, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhy J S, Krebs E G, Hakomori S, Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. - PMC - PubMed
-
- Cross F R. ‘Marker swap’ plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. - PubMed
-
- Crowther G J, Lynch D V. Characterization of sphinganine kinase activity in corn shoot microsomes. Arch Biochem Biophys. 1997;337:284–290. - PubMed
-
- De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994;219:179–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

