Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling
- PMID: 9973403
- PMCID: PMC4020419
Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling
Abstract
Stimulation of the CD95/Fas/Apo-1 receptor leads to apoptosis through activation of the caspase family of cysteine proteases and disruption of the mitochondrial transmembrane potential (Deltapsim). We show that, in Jurkat human T cells and peripheral blood lymphocytes, Fas-induced apoptosis is preceded by 1) an increase in reactive oxygen intermediates (ROI) and 2) an elevation of Deltapsim. These events are followed by externalization of phosphatidylserine (PS), disruption of Deltapsim, and cell death. The caspase inhibitor peptides, DEVD-CHO, Z-VAD.fmk, and Boc-Asp.fmk, blocked Fas-induced PS externalization, disruption of Deltapsim, and cell death, suggesting that these events are sequelae of caspase activation. By contrast, in the presence of caspase inhibitors, ROI levels and Deltapsim of Fas-stimulated cells remained elevated. Because ROI levels and Deltapsim are regulated by the supply of reducing equivalents from the pentose phosphate pathway (PPP), we studied the impact of transaldolase (TAL), a key enzyme of the PPP, on Fas signaling. Overexpression of TAL accelerated Fas-induced mitochondrial ROI production, Deltapsim elevation, activation of caspase-8 and caspase-3, proteolysis of poly(A)DP-ribose polymerase, and PS externalization. Additionally, suppression of TAL diminished these activities. Therefore, by controlling the balance between mitochondrial ROI production and metabolic supply of reducing equivalents through the PPP, TAL regulates susceptibility to Fas-induced apoptosis. Early increases in ROI levels and Deltapsim as well as the dominant effect of TAL expression on activation of caspase-8/Fas-associated death domain-like IL-1beta-converting enzyme, the most upstream member of the caspase cascade, suggest a pivotal role for redox signaling at the initiation of Fas-mediated apoptosis.
Figures

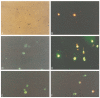

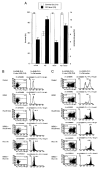

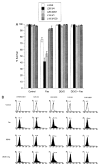
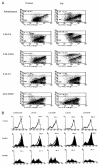


References
-
- Wyllie AH, Kerr JFR, Currie AE. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251. - PubMed
-
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355. - PubMed
-
- Itoh N, Nagata S. A novel protein domain required for apoptosis: mutational analysis of human Fas antigen. J. Biol. Chem. 1993;268:10932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
