Brominated 7-hydroxycoumarin-4-ylmethyls: photolabile protecting groups with biologically useful cross-sections for two photon photolysis
- PMID: 9990000
- PMCID: PMC15439
- DOI: 10.1073/pnas.96.4.1193
Brominated 7-hydroxycoumarin-4-ylmethyls: photolabile protecting groups with biologically useful cross-sections for two photon photolysis
Abstract
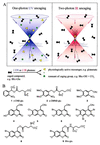
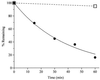
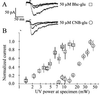
Photochemical release (uncaging) of bioactive messengers with three-dimensional spatial resolution in light-scattering media would be greatly facilitated if the photolysis could be powered by pairs of IR photons rather than the customary single UV photons. The quadratic dependence on light intensity would confine the photolysis to the focus point of the laser, and the longer wavelengths would be much less affected by scattering. However, previous caged messengers have had very small cross sections for two-photon excitation in the IR region. We now show that brominated 7-hydroxycoumarin-4-ylmethyl esters and carbamates efficiently release carboxylates and amines on photolysis, with one- and two-photon cross sections up to one or two orders of magnitude better than previously available. These advantages are demonstrated on neurons in brain slices from rat cortex and hippocampus excited by glutamate uncaged from N-(6-bromo-7-hydroxycoumarin-4-ylmethoxycarbonyl)-L-glutamate (Bhc-glu). Conventional UV photolysis of Bhc-glu requires less than one-fifth the intensities needed by one of the best previous caged glutamates, gamma-(alpha-carboxy-2-nitrobenzyl)-L-glutamate (CNB-glu). Two-photon photolysis with raster-scanned femtosecond IR pulses gives the first three-dimensionally resolved maps of the glutamate sensitivity of neurons in intact slices. Bhc-glu and analogs should allow more efficient and three-dimensionally localized uncaging and photocleavage, not only in cell biology and neurobiology but also in many technological applications.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

